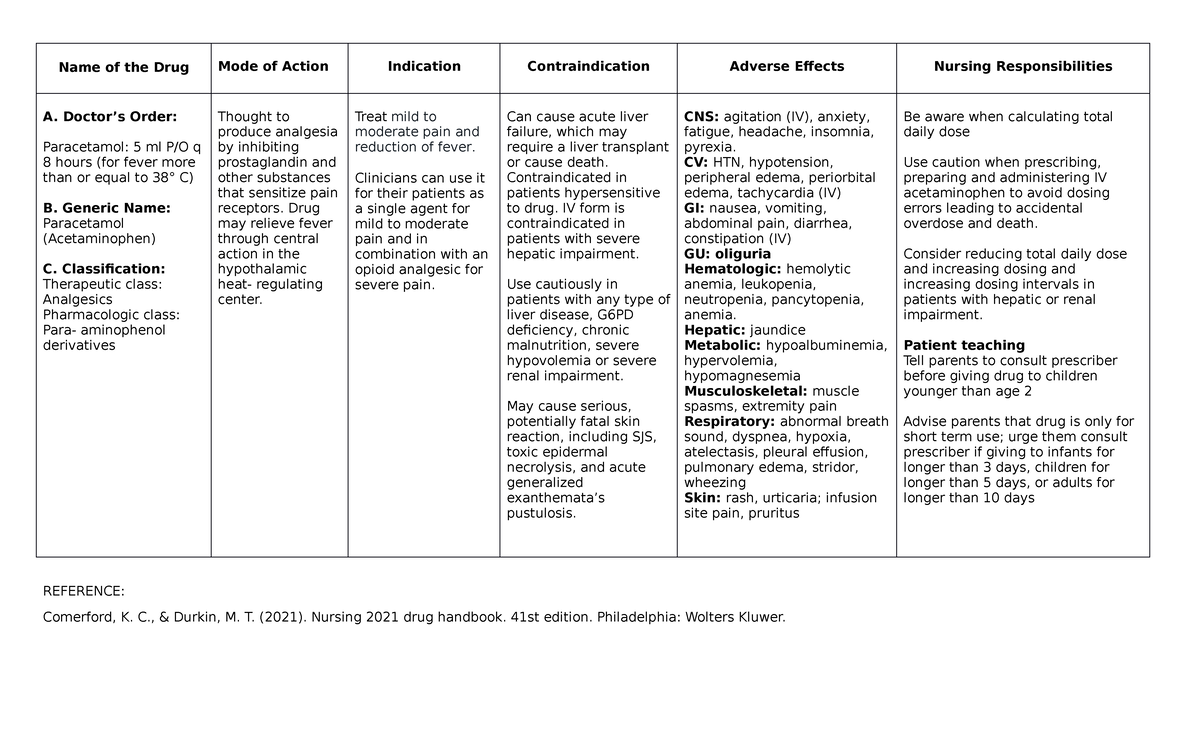
Paracetamol Iv Classification . When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Weighing less than 50 kg: Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75.
from www.studocu.com
The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Weighing less than 50 kg: When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with.
A drug study of paracetamol Pharmacy Studocu
Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Not to exceed 750 mg/dose or 3.75. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Weighing less than 50 kg: 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en.
From www.semanticscholar.org
[PDF] The Biopharmaceutical Classification System ( BCS ) Past and Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral. Paracetamol Iv Classification.
From pixels.com
Paracetamol Intravenous Drug Solution Photograph by Dr P. Marazzi Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Weighing less than 50 kg: Groups of 50 and 55 male or female (c57bl/6. Paracetamol Iv Classification.
From www.indiamart.com
Paracetamol Iv Infusion, For Hospital at Rs 546 in Chandigarh ID Paracetamol Iv Classification Not to exceed 750 mg/dose or 3.75. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Weighing less than 50 kg: Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000.. Paracetamol Iv Classification.
From www.studocu.com
Paracetamol Case study Paracetamol Generic Name Mechanism of action Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Weighing less than 50 kg: 12.5 mg/kg iv every 4 hours or. Paracetamol Iv Classification.
From www.mims.com
Aeknil Dosage/Direction for Use MIMS Philippines Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. 12.5 mg/kg iv every 4 hours. Paracetamol Iv Classification.
From tajpharma.in
Paracetamol Intravenous Infusion BP 0.5 w/v or 500mg/100ml Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Iv use. Paracetamol Iv Classification.
From pharmaceutical-journal.com
Paracetamol use in infants and young children The Pharmaceutical Journal Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Groups of 50 and 55 male. Paracetamol Iv Classification.
From webdokter.id
Paracetamol IV HJ Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Not to exceed 750 mg/dose or 3.75. The fda label for acetaminophen considers it. Paracetamol Iv Classification.
From www.studocu.com
Drug study on paracetamol (classification, action, indications Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. When. Paracetamol Iv Classification.
From hillspharmagh.com
PARACETAMOL INFUSION 10MG Hills Pharmaceuticals Ltd Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Iv use in patients with moderate. Paracetamol Iv Classification.
From www.scribd.com
An Overview of Paracetamol Including Its Classification, Indications Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Iv use in patients with moderate to severe postoperative pain reduces pain. Paracetamol Iv Classification.
From integratedlaboratories.in
Paracetamol Infusion 100ml (IntemolIV) Integrated laboratories Pvt. Ltd. Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. The fda label for acetaminophen considers it a. Paracetamol Iv Classification.
From carygetz.blogspot.com
Paracetamol Nsaids Classification CaryGetz Paracetamol Iv Classification Weighing less than 50 kg: When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at. Paracetamol Iv Classification.
From www.studypool.com
SOLUTION Paracetamol drug study Studypool Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution,. Paracetamol Iv Classification.
From rnspeak.com
Paracetamol (Biogesic) Drug Study And Nursing Considerations Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Not to exceed 750 mg/dose or 3.75. Weighing less than 50 kg:. Paracetamol Iv Classification.
From www.fiches-ide.fr
Perfalgan® (Paracétamol) Fiches IDE Paracetamol Iv Classification Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age,. Paracetamol Iv Classification.
From www.scribd.com
Paracetamol Drug Study PDF Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Weighing less than 50 kg: 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Iv use in patients with moderate to severe postoperative. Paracetamol Iv Classification.
From www.studocu.com
A drug study of paracetamol Pharmacy Studocu Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were. Paracetamol Iv Classification.
From www.tradeindia.com
Paracetamol Iv at Best Price in Panchkula, Haryana Eveson Pharma Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Not to exceed 750 mg/dose or 3.75. Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Nouvelle classification des antalgiques* (lussier & beaulieu,. Paracetamol Iv Classification.
From www.indiamart.com
Paracetamol Infusion IV, 1000 mg at Rs 79 in Surat ID 14661965688 Paracetamol Iv Classification The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Not to exceed 750 mg/dose or 3.75. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with.. Paracetamol Iv Classification.
From studylib.net
List of medication classifications Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Iv use in patients with moderate to severe postoperative pain reduces pain intensity and. Paracetamol Iv Classification.
From www.ivmpharmacia.com
Paracetamol Infusion 1000mg /100ml IVM Pharmacia Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug. Paracetamol Iv Classification.
From greencrossindia.com
Paracetamol InfusionParacetamol 1000 mg IV USE,SIDE EFFECTS,CROMOL IV Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. Weighing less than 50 kg: Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup,. Paracetamol Iv Classification.
From ep.bmj.com
Paracetamol pharmacology, prescribing and controversies ADC Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a. Paracetamol Iv Classification.
From www.sssaustralia.com.au
Paracetamol I.V Pfizer 1g x 100ml B12 RD SSS Australia SSS Paracetamol Iv Classification Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Weighing less than 50 kg: Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every. Paracetamol Iv Classification.
From pharmacyfreak.com
CLASSIFICATION OF NON STEROIDAL ANTI INFLAMMATORY DRUGS Pharmacy Freak Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this. Paracetamol Iv Classification.
From www.indiamart.com
Paracetamol Infusion IV, 1000 mg at Rs 440 in Bhopal ID 19785978962 Paracetamol Iv Classification Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Weighing less than 50 kg: Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Groups of. Paracetamol Iv Classification.
From www.ezmedlearning.com
Antibiotic Class Chart and Drug Name List Pharmacology Mnemonic — EZmed Paracetamol Iv Classification Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Nouvelle classification des antalgiques* (lussier &. Paracetamol Iv Classification.
From www.scribd.com
An Overview of Paracetamol Its Classification, Mechanism of Action Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Weighing less than 50 kg: Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice,. Paracetamol Iv Classification.
From www.studocu.com
DRUG Study Paracetamol DRUG STUDY DRUG NAME CLASSIFICATION Paracetamol Iv Classification Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. 12.5. Paracetamol Iv Classification.
From www.scribd.com
Drug Study Paracetamol Analgesic Dose (Biochemistry) Paracetamol Iv Classification Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. Not to exceed 750 mg/dose or 3.75. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Weighing less than 50. Paracetamol Iv Classification.
From sahar.ph
Paracetamol for Injection (Amgesic) Sahar Pharma Paracetamol Iv Classification Iv use in patients with moderate to severe postoperative pain reduces pain intensity and rescue opiate requirements compared with. The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug has demonstrated adverse effects in animal. Acetaminophen is available in various formulations, including tablets, capsules, syrup, oral solution, or suspension. Groups of 50 and 55 male. Paracetamol Iv Classification.
From www.sssaustralia.com.au
Paracetamol I.V Pfizer 1g x 100ml B12 RD SSS Australia SSS Paracetamol Iv Classification Weighing less than 50 kg: Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; The fda label for acetaminophen considers it a pregnancy category c drug, meaning this drug. Paracetamol Iv Classification.
From www.studocu.com
DRUG Study Paracetamol DRUG STUDY Name of Patient Baby Pearl Date of Paracetamol Iv Classification 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Not to exceed 750 mg/dose or 3.75. When intravenous paracetamol is ordered, the prescriber must review after every 48 hours of therapy to determine when a change to enteral. Acetaminophen is available in various formulations, including. Paracetamol Iv Classification.
From www.scribd.com
Paracetamol Drug Study Medicinal Chemistry Drugs Paracetamol Iv Classification Weighing less than 50 kg: Nouvelle classification des antalgiques* (lussier & beaulieu, iasp 2010) classification en. Groups of 50 and 55 male or female (c57bl/6 × c3h/he)fl (b6c3f1) mice, eight to nine weeks of age, were fed paracetamol (> 98% pure) at 3000. 12.5 mg/kg iv every 4 hours or 15 mg/kg iv every 6 hours; When intravenous paracetamol is. Paracetamol Iv Classification.