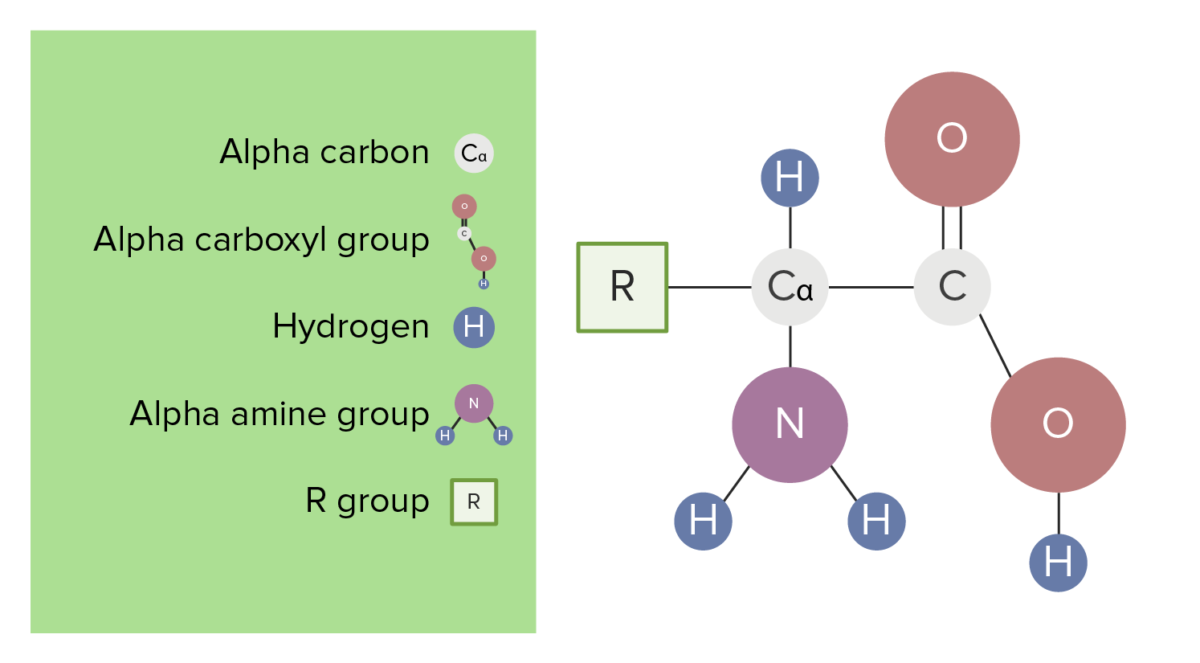
Amino Acids Are Weaker Than Carboxylic Acids Why . So amines are proton acceptors. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. Find out what is the isoelectric point and why it is not. Carboxylic acids are weak acids, therefore they are proton donors to water. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Compare the acid strengths of carboxylic acids, phenols and. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. An amino acid has both a basic amine group and an acidic carboxylic acid group. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. How to use a pka table ] should amino. Learn what amino acids are, how they are named and how they have different physical properties. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols.
from www.lecturio.com
Carboxylic acids are weak acids, therefore they are proton donors to water. Compare the acid strengths of carboxylic acids, phenols and. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. Learn what amino acids are, how they are named and how they have different physical properties. So amines are proton acceptors.
Basics of Amino Acids Concise Medical Knowledge
Amino Acids Are Weaker Than Carboxylic Acids Why So amines are proton acceptors. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. Learn what amino acids are, how they are named and how they have different physical properties. How to use a pka table ] should amino. So amines are proton acceptors. Find out what is the isoelectric point and why it is not. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. An amino acid has both a basic amine group and an acidic carboxylic acid group. Compare the acid strengths of carboxylic acids, phenols and. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Carboxylic acids are weak acids, therefore they are proton donors to water. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base.
From courses.lumenlearning.com
Amino Acids Structure Nutrition Amino Acids Are Weaker Than Carboxylic Acids Why Find out what is the isoelectric point and why it is not. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. Learn what amino acids are, how they are named and how they have different physical properties. Learn how to measure the acidity of. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.masterorganicchemistry.com
Simplifying the reactions of carboxylic acid derivatives (part 1 Amino Acids Are Weaker Than Carboxylic Acids Why Carboxylic acids are weak acids, therefore they are proton donors to water. Compare the acid strengths of carboxylic acids, phenols and. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. How to use a pka table ] should amino. Learn what amino acids are, how. Amino Acids Are Weaker Than Carboxylic Acids Why.
From courses.lumenlearning.com
Proteins OpenStax Biology 2e Amino Acids Are Weaker Than Carboxylic Acids Why Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Compare the acid strengths of carboxylic acids, phenols and. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Carboxylic acids are weak acids, therefore they are proton donors to water. An amino acid has both. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.chemistrysteps.com
Naming Carboxylic Acids Chemistry Steps Amino Acids Are Weaker Than Carboxylic Acids Why An amino acid has both a basic amine group and an acidic carboxylic acid group. How to use a pka table ] should amino. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Although much. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.coursehero.com
[Solved] Give the 20 amino acids and give their functions. Course Hero Amino Acids Are Weaker Than Carboxylic Acids Why Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Find out. Amino Acids Are Weaker Than Carboxylic Acids Why.
From socratic.org
How does pH affect amino acid structure? + Example Amino Acids Are Weaker Than Carboxylic Acids Why Compare the acid strengths of carboxylic acids, phenols and. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Find out what is the isoelectric point and why it is not. Carboxylic acids are weak acids, therefore they are proton donors to water. Learn about the general structure, classification, and properties of amino. Amino Acids Are Weaker Than Carboxylic Acids Why.
From slidetodoc.com
Amino acids peptides and proteins Properties of Amino Amino Acids Are Weaker Than Carboxylic Acids Why An amino acid has both a basic amine group and an acidic carboxylic acid group. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. So amines are proton acceptors. Learn about the general structure, classification, and properties of amino acids, the building blocks of. Amino Acids Are Weaker Than Carboxylic Acids Why.
From byjus.com
Carboxylic Acids Properties, Nomenclature & Uses Chemistry Byju's Amino Acids Are Weaker Than Carboxylic Acids Why How to use a pka table ] should amino. Carboxylic acids are weak acids, therefore they are proton donors to water. Learn what amino acids are, how they are named and how they have different physical properties. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Learn about the general structure, classification,. Amino Acids Are Weaker Than Carboxylic Acids Why.
From mavink.com
Amino Acid Structure Labeled Amino Acids Are Weaker Than Carboxylic Acids Why Learn what amino acids are, how they are named and how they have different physical properties. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. An amino acid has both a basic amine group and an acidic carboxylic acid group. Learn how to measure the acidity of organic acids using pka values. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.researchgate.net
Amino acids and peptide bonds (A) Amino acids consist of a carbon atom Amino Acids Are Weaker Than Carboxylic Acids Why An amino acid has both a basic amine group and an acidic carboxylic acid group. Find out what is the isoelectric point and why it is not. Carboxylic acids are weak acids, therefore they are proton donors to water. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. This is a favorable. Amino Acids Are Weaker Than Carboxylic Acids Why.
From dxorxsjrp.blob.core.windows.net
Amino Acid Composition Standard at Mark Howell blog Amino Acids Are Weaker Than Carboxylic Acids Why Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Learn what amino acids are, how they are named and how they have different physical properties. Carboxylic acids are weak acids, therefore they are proton donors to water. Compare the acid strengths of carboxylic acids, phenols. Amino Acids Are Weaker Than Carboxylic Acids Why.
From klamzjcfj.blob.core.windows.net
Examples Of Amino Acid Derivatives at Juliana Noland blog Amino Acids Are Weaker Than Carboxylic Acids Why Carboxylic acids are weak acids, therefore they are proton donors to water. Compare the acid strengths of carboxylic acids, phenols and. An amino acid has both a basic amine group and an acidic carboxylic acid group. Find out what is the isoelectric point and why it is not. Find out why amino acids (apart from glycine) are chiral and how. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.thesciencehive.co.uk
Carboxylic acids and esters — the science hive Amino Acids Are Weaker Than Carboxylic Acids Why Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Find out what is the isoelectric point and why it is not. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. Learn how amino acids behave in. Amino Acids Are Weaker Than Carboxylic Acids Why.
From loeoxecye.blob.core.windows.net
Chemical Acids List at Maria Carr blog Amino Acids Are Weaker Than Carboxylic Acids Why How to use a pka table ] should amino. Find out what is the isoelectric point and why it is not. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Carboxylic acids are weak acids, therefore they are proton donors to water. So amines are proton acceptors. Find out why amino acids. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.slideserve.com
PPT Chapter 19 Carboxylic Acids PowerPoint Presentation, free Amino Acids Are Weaker Than Carboxylic Acids Why How to use a pka table ] should amino. Find out what is the isoelectric point and why it is not. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Carboxylic acids are weak acids, therefore they are proton donors to water. An amino acid has both a basic amine group and. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.brainkart.com
Amino Acids Can Act as Both Acids and Bases Amino Acids Are Weaker Than Carboxylic Acids Why Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. So amines are proton acceptors. How to use a pka table ] should amino. Carboxylic acids are weak acids, therefore they are proton donors to water. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. An. Amino Acids Are Weaker Than Carboxylic Acids Why.
From chem.libretexts.org
17.6 Relative Reactivities of Carboxylic Acids and Carboxylic Acid Amino Acids Are Weaker Than Carboxylic Acids Why So amines are proton acceptors. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. An amino acid has both a basic amine group and an acidic carboxylic. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.slideserve.com
PPT Carboxylic Acids and Carboxylic Acid Derivatives PowerPoint Amino Acids Are Weaker Than Carboxylic Acids Why Learn what amino acids are, how they are named and how they have different physical properties. Find out what is the isoelectric point and why it is not. An amino acid has both a basic amine group and an acidic carboxylic acid group. Learn how to measure the acidity of organic acids using pka values and how the nature of. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.researchgate.net
4. Two amino acids (the carboxylicgroup from the amino acid 1 and the Amino Acids Are Weaker Than Carboxylic Acids Why Learn what amino acids are, how they are named and how they have different physical properties. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. How to use a pka table ] should amino. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Find out. Amino Acids Are Weaker Than Carboxylic Acids Why.
From chemistry.com.pk
A Brief Introduction of Amino Acids The Building Blocks of Proteins Amino Acids Are Weaker Than Carboxylic Acids Why Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Carboxylic acids are weak acids, therefore they are proton donors to water. How to use a pka table ] should amino. Compare the acid strengths of carboxylic acids, phenols and. An amino acid has both a basic amine group and an acidic carboxylic. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.chegg.com
Solved d) Explain why peroxyacids are weaker than carboxylic Amino Acids Are Weaker Than Carboxylic Acids Why Find out what is the isoelectric point and why it is not. Learn what amino acids are, how they are named and how they have different physical properties. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. So amines are proton acceptors. Although much weaker. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.chemistrysteps.com
Organic Acids and Bases Chemistry Steps Amino Acids Are Weaker Than Carboxylic Acids Why Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. An amino acid has both a basic amine group and an acidic carboxylic acid group. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Learn how to measure the acidity of organic acids using pka values. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.expii.com
Amino Acids — Overview & Structure Expii Amino Acids Are Weaker Than Carboxylic Acids Why An amino acid has both a basic amine group and an acidic carboxylic acid group. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Carboxylic acids are weak acids, therefore they are proton donors to water. This is a favorable equilibrium because the reaction proceeds. Amino Acids Are Weaker Than Carboxylic Acids Why.
From slideplayer.com
Types of Chemical Reactions and Solution Stoichiometry ppt download Amino Acids Are Weaker Than Carboxylic Acids Why Compare the acid strengths of carboxylic acids, phenols and. Carboxylic acids are weak acids, therefore they are proton donors to water. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. Although much weaker than mineral acids,. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Amino Acids Are Weaker Than Carboxylic Acids Why How to use a pka table ] should amino. Learn what amino acids are, how they are named and how they have different physical properties. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. An. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.lecturio.com
Basics of Amino Acids Concise Medical Knowledge Amino Acids Are Weaker Than Carboxylic Acids Why Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. So amines are proton acceptors. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.medschoolcoach.com
Amino Acid Classification MCAT Biochemistry MedSchoolCoach Amino Acids Are Weaker Than Carboxylic Acids Why Learn what amino acids are, how they are named and how they have different physical properties. Find out what is the isoelectric point and why it is not. An amino acid has both a basic amine group and an acidic carboxylic acid group. How to use a pka table ] should amino. So amines are proton acceptors. This is a. Amino Acids Are Weaker Than Carboxylic Acids Why.
From wou.edu
Chapter 2 Protein Structure Chemistry Amino Acids Are Weaker Than Carboxylic Acids Why How to use a pka table ] should amino. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. Learn what amino acids are, how they are named and how they have different physical properties. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Learn how. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.thesciencehive.co.uk
Amines, amides and amino acids — the science hive Amino Acids Are Weaker Than Carboxylic Acids Why Learn what amino acids are, how they are named and how they have different physical properties. Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. So amines are proton acceptors. Carboxylic acids are weak acids, therefore they are proton donors to water. Find out why. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.echemi.com
Why are carboxylic acids stronger acid than phenol, alcohol, and water Amino Acids Are Weaker Than Carboxylic Acids Why An amino acid has both a basic amine group and an acidic carboxylic acid group. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. How to use a pka table ] should amino. Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. This is. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.youtube.com
why phenols are weaker acids than carboxylic acids II Chemistry Capsule Amino Acids Are Weaker Than Carboxylic Acids Why Learn how amino acids behave in aqueous solutions as they change ph by adding acids or alkalis. Learn what amino acids are, how they are named and how they have different physical properties. Although much weaker than mineral acids, carboxylic acids are nevertheless much stronger acids than alcohols and phenols. Compare the acid strengths of carboxylic acids, phenols and. Find. Amino Acids Are Weaker Than Carboxylic Acids Why.
From byjus.com
Why carboxylic acid is stronger acid then phenol? Or Why the conjugated Amino Acids Are Weaker Than Carboxylic Acids Why Compare the acid strengths of carboxylic acids, phenols and. This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. So amines are proton acceptors. Find out what is the isoelectric point and why it is not. Learn about the general structure, classification, and properties of. Amino Acids Are Weaker Than Carboxylic Acids Why.
From www.animalia-life.club
Amino Group And Carboxyl Group Amino Acids Are Weaker Than Carboxylic Acids Why Learn how to measure the acidity of organic acids using pka values and how the nature of the anion formed affects the ionisation equilibrium. Learn what amino acids are, how they are named and how they have different physical properties. So amines are proton acceptors. An amino acid has both a basic amine group and an acidic carboxylic acid group.. Amino Acids Are Weaker Than Carboxylic Acids Why.
From basicmedicalkey.com
Amino Acids in Proteins Basicmedical Key Amino Acids Are Weaker Than Carboxylic Acids Why This is a favorable equilibrium because the reaction proceeds from a stronger acid and a stronger base to a weaker acid and a weaker base. Learn what amino acids are, how they are named and how they have different physical properties. Find out why amino acids (apart from glycine) are chiral and how they rotate plane polarised light. An amino. Amino Acids Are Weaker Than Carboxylic Acids Why.
From aminoco.com
Acidic and Basic Amino Acids Explained The Amino Company Amino Acids Are Weaker Than Carboxylic Acids Why Compare the acid strengths of carboxylic acids, phenols and. Learn about the general structure, classification, and properties of amino acids, the building blocks of proteins. How to use a pka table ] should amino. So amines are proton acceptors. Carboxylic acids are weak acids, therefore they are proton donors to water. An amino acid has both a basic amine group. Amino Acids Are Weaker Than Carboxylic Acids Why.