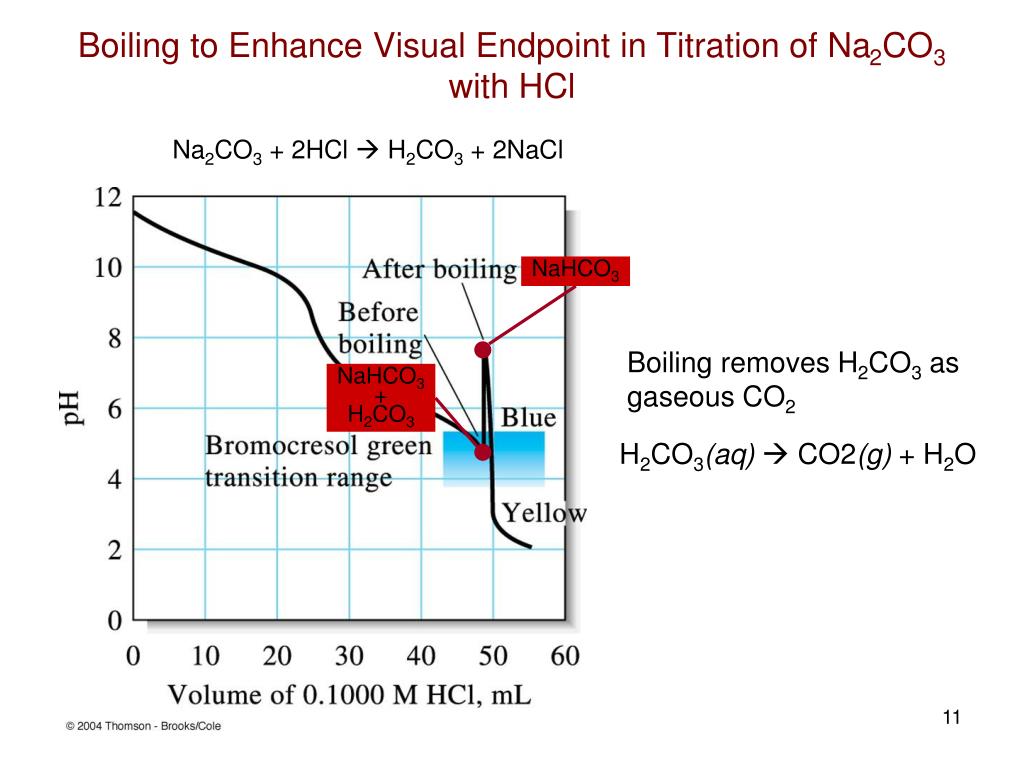
Hcl Vs Sodium Carbonate Titration . A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. This process is known as standardising the hydrochloric acid. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint.
from www.slideserve.com
Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is known as standardising the hydrochloric acid. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3.
PPT Titration of Sodium Carbonate PowerPoint Presentation, free
Hcl Vs Sodium Carbonate Titration A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. This process is known as standardising the hydrochloric acid.
From www.youtube.com
Titration of Concentrated HCl by Standard Sodium Carbonate Solution Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration This process is known as standardising the hydrochloric acid. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. Today, you will use it to find the concentration. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
Titration of HCl with standard solution of sodium carbonate. YouTube Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue. Hcl Vs Sodium Carbonate Titration.
From www.trunorthink.com
Carbonate Chemistry Applied To The Productions Of Still Water Hcl Vs Sodium Carbonate Titration This process is known as standardising the hydrochloric acid. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Acidbase titrations PowerPoint Presentation, free download ID Hcl Vs Sodium Carbonate Titration A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Today, you will use it. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Titration hcl vs sodium carbonate Studypool Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The color of the solution changes in this titration process due to the formation of the product which is. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
HCl + Sodium Carbonate Titration Maths Overview YouTube Hcl Vs Sodium Carbonate Titration A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. This process is known as standardising the hydrochloric acid. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and. Hcl Vs Sodium Carbonate Titration.
From www.chegg.com
Solved Procedure for Titration of HCl and Sodium Carbonate Hcl Vs Sodium Carbonate Titration The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. This. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Sodium carbonate titrationhcl with hcl na2co3 Studypool Hcl Vs Sodium Carbonate Titration Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. The color of the solution changes in this titration process due to the formation of the product which is. Hcl Vs Sodium Carbonate Titration.
From www.scribd.com
Standardization of Hydrochloric Acid Sodium Carbonate Titration Hcl Vs Sodium Carbonate Titration A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Today, you will use it to find the concentration. Hcl Vs Sodium Carbonate Titration.
From www.tessshebaylo.com
Chemical Equation For Sodium Carbonate And Hydrochloric Acid Tessshebaylo Hcl Vs Sodium Carbonate Titration Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. In this experiment a solution of hydrochloric acid. Hcl Vs Sodium Carbonate Titration.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Hcl Vs Sodium Carbonate Titration This process is known as standardising the hydrochloric acid. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. The hydrochloric acid solution is generally titrated by using. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Titration hcl vs sodium carbonate Studypool Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is known as standardising the hydrochloric acid. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The color of the solution changes in this titration process due to the formation of. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. The color of. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
Titration Hydrochloric Acid vs Sodium Carbonate YouTube Hcl Vs Sodium Carbonate Titration In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. This process is known as standardising the hydrochloric acid. A titration’s end point was determined using litmus as an indicator, which is red. Hcl Vs Sodium Carbonate Titration.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Hcl Vs Sodium Carbonate Titration This process is known as standardising the hydrochloric acid. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Today, you will use it to find the concentration of. Hcl Vs Sodium Carbonate Titration.
From www.tessshebaylo.com
Balanced Chemical Equation Between Sodium Carbonate And Hydrochloric Hcl Vs Sodium Carbonate Titration In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The hydrochloric acid solution is generally titrated by. Hcl Vs Sodium Carbonate Titration.
From www.scribd.com
HCL VS Sodium Carbonate 2 PDF Titration Chemistry Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. This process is known as standardising the hydrochloric acid. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,.. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. A. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT Titration of Sodium Carbonate PowerPoint Presentation, free Hcl Vs Sodium Carbonate Titration Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. A titration’s end point. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Sodium carbonate titrationhcl with hcl na2co3 Studypool Hcl Vs Sodium Carbonate Titration The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The hydrochloric acid solution is generally titrated. Hcl Vs Sodium Carbonate Titration.
From www.scribd.com
P4 Titration Sodium Carbonate Vs HCL PDF Hcl Vs Sodium Carbonate Titration A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The color of the solution changes in this. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
DOUBLE INDICATOR TITRATION part 01Mixture of Sodium Carbonate and Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. A titration’s. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
titration of sodium carbonate with HCI titration of Na2CO3 Vs HCI Hcl Vs Sodium Carbonate Titration Today, you will use it to find the concentration of dilute hydrochloric acid by titration. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Titration hcl vs sodium carbonate Studypool Hcl Vs Sodium Carbonate Titration This process is known as standardising the hydrochloric acid. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. The hydrochloric acid solution is generally titrated by using. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
11 sci practical Titration of Sodium carbonate and HCl YouTube Hcl Vs Sodium Carbonate Titration The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. A. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
Lab 4.... HCl with Sodium carbonate titration YouTube Hcl Vs Sodium Carbonate Titration A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The hydrochloric acid solution is generally titrated by using. Hcl Vs Sodium Carbonate Titration.
From www.slideserve.com
PPT ACIDBASE INDICATORS PowerPoint Presentation, free download ID Hcl Vs Sodium Carbonate Titration A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. This process is known as standardising the hydrochloric acid. In. Hcl Vs Sodium Carbonate Titration.
From www.scribd.com
Titration (Sodium Carbonate and HCL) PDF Hcl Vs Sodium Carbonate Titration The hydrochloric acid solution is generally titrated by using a methyl orange indicator against the prepared standard sodium carbonate solution. Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. A comprehensive guide. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
Sodium Carbonate vs HCl Titration YouTube Hcl Vs Sodium Carbonate Titration Today, you will use it to find the concentration of dilute hydrochloric acid by titration. The color of the solution changes in this titration process due to the formation of the product which is called the endpoint. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or. Hcl Vs Sodium Carbonate Titration.
From www.youtube.com
DOUBLE INDICATOR TITRATION Part 02 Mixture of Sodium Carbonate and Hcl Vs Sodium Carbonate Titration In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. A titration’s end point was determined using litmus as an indicator, which is red in acidic solutions and blue in basic solutions, or by the cessation of co 2 effervescence when neutralizing co2 − 3. The color of the. Hcl Vs Sodium Carbonate Titration.
From stevenemahurino.blob.core.windows.net
Acid Base Titration Na2Co3 And Hcl at stevenemahurino blog Hcl Vs Sodium Carbonate Titration In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. The color. Hcl Vs Sodium Carbonate Titration.
From www.studypool.com
SOLUTION Titration na2co3 vs hcl Studypool Hcl Vs Sodium Carbonate Titration A comprehensive guide on conducting a titration of hydrochloric acid against standard sodium carbonate, including aim,. In this experiment a solution of hydrochloric acid will be standardized against pure sodium carbonate and then used to determine the. Determination of strength of a given solution of dilute hydrochloric acid by titrating it against standard solution of sodium carbonate solution. This process. Hcl Vs Sodium Carbonate Titration.