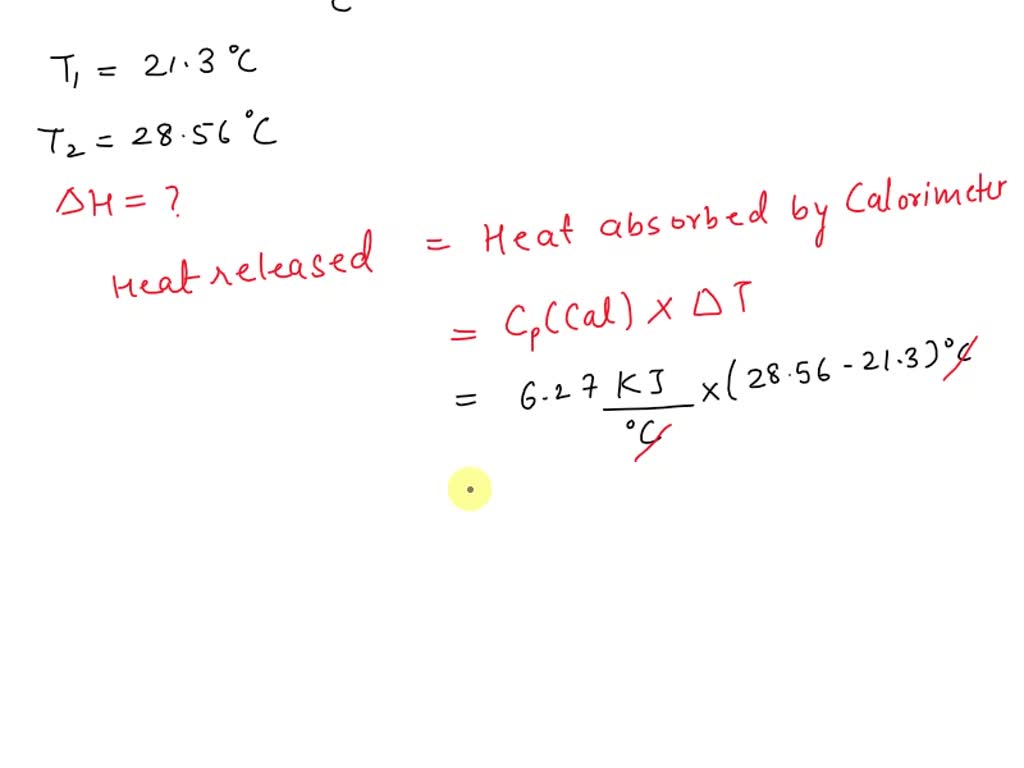A Calorimeter Has A Heat Capacity Of 1265 . Click here to get an answer to your question: A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j//g c. A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265j/^circ c. A calorimeter has specific heat capacity of 1265 j/g°c. A calorimeter has a heat capacity of 1265 j/oc. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. This equation binds temperature change and heat: A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c.
from www.numerade.com
A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A calorimeter has a heat capacity of 1265j//g c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j/^circ c. This equation binds temperature change and heat: A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A reaction causes the temperature of the. A calorimeter has specific heat capacity of 1265 j/g°c. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. Click here to get an answer to your question:
SOLVED A calorimeter has measured a heat capacity of 6.27 kJ/°C. The
A Calorimeter Has A Heat Capacity Of 1265 This equation binds temperature change and heat: A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265j//g c. This equation binds temperature change and heat: Click here to get an answer to your question: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A calorimeter has specific heat capacity of 1265 j/g°c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A calorimeter has a heat capacity of 1265j/^circ c.
From www.numerade.com
SOLVED A calorimeter has measured a heat capacity of 6.27 kJ/°C. The A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temperature of. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this A Calorimeter Has A Heat Capacity Of 1265 Click here to get an answer to your question: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c. A Calorimeter Has A Heat Capacity Of 1265.
From chemistrytalk.org
Calorimetry ChemTalk A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265 j/oc. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j//g c. Click here to get an answer to your question: A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A reaction causes the. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved 5. A calorimeter has a heat capacity of 35.45 J/∘C. A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j//g c. Click here to get an answer to your question: A. A Calorimeter Has A Heat Capacity Of 1265.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube A Calorimeter Has A Heat Capacity Of 1265 If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A reaction causes the temperature of the. Click here to get an answer to your question: A calorimeter has specific heat capacity of 1265 j/g°c. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A calorimeter has a heat capacity of. A Calorimeter Has A Heat Capacity Of 1265.
From www.toppr.com
In a constant volume calorimeter, 3.5 g of a gas with molecular weight A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A calorimeter has specific heat capacity of 1265 j/g°c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A calorimeter has a heat capacity of 1265j//g c. A calorimeter has a heat capacity of 1265j/^circ c. A calorimeter has a. A Calorimeter Has A Heat Capacity Of 1265.
From www.animalia-life.club
Calorimeter Diagram A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j/^circ c. A calorimeter has a heat capacity of 1265j//g c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A calorimeter. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved ACTIVITY 1 A Heat Capacity of the Calorimeter 1. A Calorimeter Has A Heat Capacity Of 1265 \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265 j/oc. This equation binds temperature change and heat: A calorimeter has specific heat. A Calorimeter Has A Heat Capacity Of 1265.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards A Calorimeter Has A Heat Capacity Of 1265 The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. This equation binds temperature change and heat: A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265 j/oc. A. A Calorimeter Has A Heat Capacity Of 1265.
From saylordotorg.github.io
Calorimetry A Calorimeter Has A Heat Capacity Of 1265 The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has a heat capacity of 1265j//g c. A calorimeter has specific heat capacity of 1265 j/g°c. This equation binds temperature change and heat: Click here to get an answer. A Calorimeter Has A Heat Capacity Of 1265.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A calorimeter has specific heat capacity of 1265 j/g°c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved Calculate Heat capacity of the calorimeter (J/ ºC) , A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265j/^circ c. A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265j//g c. This equation binds temperature change and heat: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the. A Calorimeter Has A Heat Capacity Of 1265.
From www.youtube.com
050 Calorimetry YouTube A Calorimeter Has A Heat Capacity Of 1265 \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. Click here to get an answer to your question: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the. This equation binds temperature change and heat: A reaction causes the. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
A 0.5865 g sample of lactic acid (HC3H5O3) reacts with oxygen in a A Calorimeter Has A Heat Capacity Of 1265 This equation binds temperature change and heat: \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. Click here to get an answer to your question: A calorimeter has a heat capacity of 1265j//g c. A reaction causes the temperature of the calorimeter. A Calorimeter Has A Heat Capacity Of 1265.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. This equation binds temperature change and heat: A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. Click here to get an answer to your question: If $\pu{1.25 g}$. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
SOLVED 'A 89.2 g piece of aluminum (which has molar heat capacity of A Calorimeter Has A Heat Capacity Of 1265 \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. This equation binds temperature change and heat: A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265j//g c. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved A bomb calorimeter has a heat capacity of 2.47 kJ/K. A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. Click here to get an answer to your question: A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. This equation binds temperature change and heat: A reaction causes the temperature of the. If $\pu{1.25 g}$ of glucose are burnt. A Calorimeter Has A Heat Capacity Of 1265.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has. A Calorimeter Has A Heat Capacity Of 1265.
From studylib.net
Heat Capacity of Metals PreLab A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265j/^circ c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A calorimeter has specific heat capacity of 1265 j/g°c. A calorimeter has a heat capacity of 1265j//g c. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temp of. A Calorimeter Has A Heat Capacity Of 1265.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265j/^circ c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j//g c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A reaction causes the temperature of the calorimeter to change from 22.34oc to. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
SOLVED A 0.43 g sample of glucose (MW = 180. g/mol) is burned in a A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j//g c. This equation binds temperature change and heat: A reaction causes the temperature. A Calorimeter Has A Heat Capacity Of 1265.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data A Calorimeter Has A Heat Capacity Of 1265 If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265j/^circ c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34 c. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the. Click here to get an answer to your question: A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved Combustion (bomb) calorimeter. In an experiment, a A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. This equation binds temperature change and heat: Click here to get an answer to your question: A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has specific heat. A Calorimeter Has A Heat Capacity Of 1265.
From users.highland.edu
Calorimetry A Calorimeter Has A Heat Capacity Of 1265 \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. This equation binds temperature change and heat: A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has a heat capacity of 1265j/^circ c. If $\pu{1.25 g}$ of glucose are burnt in a. A Calorimeter Has A Heat Capacity Of 1265.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265j/^circ c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. Click here to get an answer to your question: This equation binds temperature change and heat: A calorimeter has specific heat capacity of. A Calorimeter Has A Heat Capacity Of 1265.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube A Calorimeter Has A Heat Capacity Of 1265 If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265j/^circ c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
SOLVED Cyclohexanol, C6H12O, has a heat of combustion of 890.7 kcal A Calorimeter Has A Heat Capacity Of 1265 The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A calorimeter has a heat capacity of 1265j/^circ c. A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265 j/oc. \scriptsize \delta q = m \cdot c \cdot. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
SOLVED Can somebody explain how to find the heat absorbed by a A Calorimeter Has A Heat Capacity Of 1265 Click here to get an answer to your question: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A reaction causes the temperature of the. A calorimeter has a heat capacity of 1265j//g c. A calorimeter has a heat capacity of 1265. A Calorimeter Has A Heat Capacity Of 1265.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download A Calorimeter Has A Heat Capacity Of 1265 A reaction causes the temperature of the calorimeter to change from 22.34 c to 25.12 c. A calorimeter has a heat capacity of 1265 j/oc. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. This equation binds temperature change and heat: A calorimeter has. A Calorimeter Has A Heat Capacity Of 1265.
From www.numerade.com
A 0.1375g sample of solid magnesium is burned in a constantvolume A Calorimeter Has A Heat Capacity Of 1265 Click here to get an answer to your question: The combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has a heat capacity of 1265j/^circ c. A calorimeter has specific heat capacity of 1265 j/g°c. A reaction causes the temperature of the calorimeter to change from. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has a heat capacity of 1265j//g c. \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. A reaction causes the temperature of the. A calorimeter has specific heat capacity of 1265 j/g°c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c.. A Calorimeter Has A Heat Capacity Of 1265.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry A Calorimeter Has A Heat Capacity Of 1265 If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. A calorimeter has specific heat capacity of 1265 j/g°c. Click here to get an answer to your question: A reaction causes the temperature. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or A Calorimeter Has A Heat Capacity Of 1265 \scriptsize \delta q = m \cdot c \cdot \delta t δq=m⋅c⋅δt. This equation binds temperature change and heat: A calorimeter has a heat capacity of 1265j/^circ c. A calorimeter has a heat capacity of 1265 j/oc. A calorimeter has a heat capacity of 1265j//g c. Click here to get an answer to your question: A reaction causes the temperature of. A Calorimeter Has A Heat Capacity Of 1265.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, A Calorimeter Has A Heat Capacity Of 1265 A calorimeter has a heat capacity of 1265j/^circ c. A reaction causes the temp of the 50g calorimeter to change from 22.34°c to 25.12°c. If $\pu{1.25 g}$ of glucose are burnt in a calorimeter containing $\pu{0.95 kg}$ of. A calorimeter has specific heat capacity of 1265 j/g°c. A calorimeter has a heat capacity of 1265 j/oc. A reaction causes the. A Calorimeter Has A Heat Capacity Of 1265.