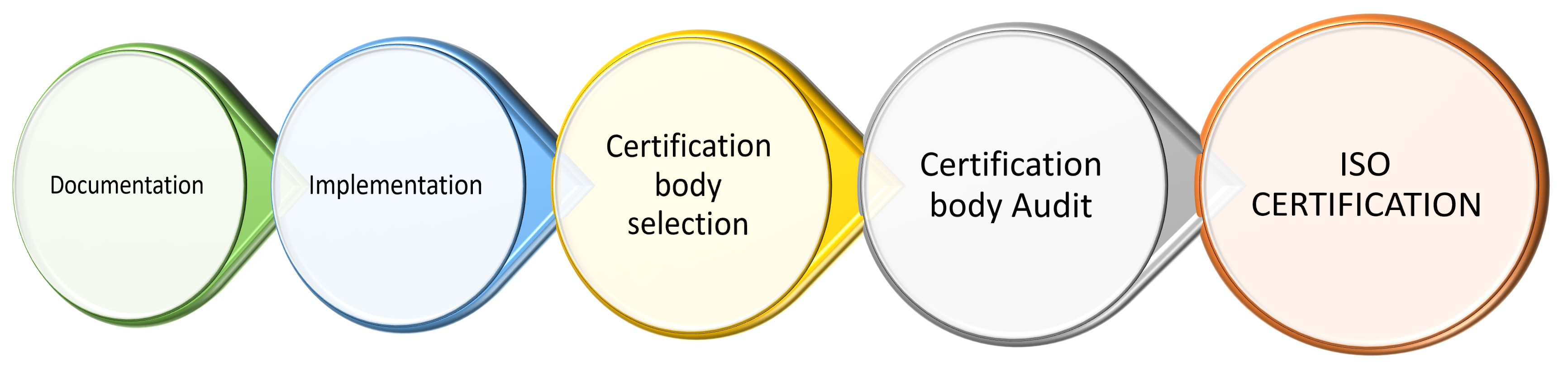
Cap Iso Certification . This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Why did the cap launch the accreditation. College of american pathologists (cap) ngsp certificate of traceability. Accreditation is a continual process. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. How long has the cap been accrediting to iso 15189? By the end of this period,. The cap’s iso 15189 accreditation program. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Things to know for cap laboratory accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. Ten steps to cap laboratory accreditation.
from www.iso-certification.us
College of american pathologists (cap) ngsp certificate of traceability. Ten steps to cap laboratory accreditation. By the end of this period,. How long has the cap been accrediting to iso 15189? Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. Accreditation is a continual process. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Things to know for cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard.
ISO Certification FAQ ISOCERTIFICATION.US
Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. How long has the cap been accrediting to iso 15189? Accreditation is a continual process. Things to know for cap laboratory accreditation. By the end of this period,. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. Why did the cap launch the accreditation. The cap’s iso 15189 accreditation program. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Ten steps to cap laboratory accreditation. College of american pathologists (cap) ngsp certificate of traceability. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard.
From www.indiamart.com
ISO Certification Service, ISO सर्टिफिकेशन सर्विस, आईएसओ सर्टिफिकेशन Cap Iso Certification How long has the cap been accrediting to iso 15189? Ten steps to cap laboratory accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. The cap’s iso 15189 accreditation. Cap Iso Certification.
From www.aruplab.com
ARUP Announces Successful Completion of CAP ISO 15189 Assessment ARUP Cap Iso Certification By the end of this period,. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Why did the cap launch the accreditation. How long has the cap been accrediting to iso 15189? Things to. Cap Iso Certification.
From bestisocert.blogspot.com
Best ISO Certification Provide ISO Certification Services Identify Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Accreditation is a continual process. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. How long has the cap been accrediting to iso 15189? Ten steps to cap laboratory accreditation. Why did the cap launch the accreditation. Things to know for cap laboratory accreditation. Learn more about. Cap Iso Certification.
From vskandco.com
ISO Certification Consultants VSK & Co ADVOCATES & LEGAL CONSULTANTS Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Ten steps to cap laboratory accreditation. Why did the cap launch the accreditation. The cap’s iso 15189 accreditation program. College of american pathologists (cap) ngsp certificate of traceability. By the end of this period,. Accreditation is a continual process. How long has the cap been accrediting to iso 15189? This program delivers the prescriptive. Cap Iso Certification.
From ymbc.edu.in
» ISO Certification Cap Iso Certification The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Ten steps to cap laboratory accreditation. Things to know for cap laboratory accreditation. College of american pathologists (cap) ngsp certificate of traceability. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Why did the cap launch the accreditation. The cap’s iso 15189 accreditation program.. Cap Iso Certification.
From www.aeon.co.th
ระบบคุณภาพที่ได้รับการรับรอง บริษัท อิออน ธนสินทรัพย์ (ไทยแลนด์ Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Ten steps to cap laboratory accreditation. College of american pathologists (cap) ngsp certificate of traceability. By the end of this period,. How long has the cap been accrediting to iso 15189? Learn more about our. Cap Iso Certification.
From sattvacfo.com
ISO Certification in India Full Form & Meaning Cap Iso Certification How long has the cap been accrediting to iso 15189? Ten steps to cap laboratory accreditation. Things to know for cap laboratory accreditation. Why did the cap launch the accreditation. By the end of this period,. Accreditation is a continual process. The cap’s iso 15189 accreditation program. Learn more about our global network of fully cap accredited, iso 15189 central. Cap Iso Certification.
From www.desiretech.com
ISO 90012015 Certification Best site Design Development Company Cap Iso Certification Why did the cap launch the accreditation. How long has the cap been accrediting to iso 15189? The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Accreditation is a continual process. By the end. Cap Iso Certification.
From www.alamy.com
Set of ISO Certification stamp and labels. ISO Certified badge Cap Iso Certification By the end of this period,. Why did the cap launch the accreditation. How long has the cap been accrediting to iso 15189? Ten steps to cap laboratory accreditation. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Learn more about our global network of fully cap accredited, iso 15189. Cap Iso Certification.
From www.thaidigitalid.com
Certificate of ISO 27001 Thai Digital ID Company Limited Cap Iso Certification Ten steps to cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Accreditation is a continual process. The cap’s iso 15189 accreditation program. Things to know for cap laboratory accreditation. How long has the cap been accrediting to iso 15189? College of american pathologists (cap) ngsp certificate of traceability. Learn. Cap Iso Certification.
From cerbaresearch.com
CerbACT Asia receives CAP Certification Cerba Research Cap Iso Certification Ten steps to cap laboratory accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Accreditation is a continual process. This program delivers the prescriptive technical requirements. Cap Iso Certification.
From www.dreamstime.com
ISO CERTIFICATION Written Word on Red Stamp Sign Stock Illustration Cap Iso Certification By the end of this period,. Ten steps to cap laboratory accreditation. Accreditation is a continual process. College of american pathologists (cap) ngsp certificate of traceability. Why did the cap launch the accreditation. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Things to. Cap Iso Certification.
From www.youtube.com
Iso certification registration online Iso certificate registration Cap Iso Certification By the end of this period,. The cap’s iso 15189 accreditation program. Why did the cap launch the accreditation. Things to know for cap laboratory accreditation. Ten steps to cap laboratory accreditation. Accreditation is a continual process. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施. Cap Iso Certification.
From aegis.qa
Understand ISO 9001 Certification Top Consultant for ISO Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. Accreditation is a continual process. The cap’s iso 15189 accreditation program. Ten steps to cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the. Cap Iso Certification.
From qfscerts.com
Essential ISO Certification services Guide QFS Certs Cap Iso Certification Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. By the end of this period,. Accreditation is a continual process. Ten steps to cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. How long has the. Cap Iso Certification.
From www.indiamart.com
Iso Certification Registration Services at Rs 7000/year iso 9001 2008 Cap Iso Certification By the end of this period,. Why did the cap launch the accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. The cap’s iso 15189 accreditation program. Things to. Cap Iso Certification.
From bevaglobal.com
Expert ISO Certification Guidance BEVA Global Management, Ottawa Cap Iso Certification The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Things to know for cap laboratory accreditation. The cap’s iso 15189 accreditation program. Ten steps to cap laboratory accreditation. Why did the cap launch the accreditation. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. This program delivers the prescriptive technical requirements established in. Cap Iso Certification.
From www.dreamstime.com
Iso Certification Seal. Stamp Stock Vector Illustration of round Cap Iso Certification The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Things to know for cap laboratory accreditation. Ten steps to cap laboratory accreditation. Why did the cap launch the accreditation. College of american pathologists (cap) ngsp certificate of traceability. By the end of this period,. This program delivers the prescriptive technical. Cap Iso Certification.
From www.sambhavcaps.com
Sambhav Cap Creations • Services Cap Iso Certification College of american pathologists (cap) ngsp certificate of traceability. Things to know for cap laboratory accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. How long has the cap been accrediting to iso 15189? By the end of this period,. Accreditation is a continual process.. Cap Iso Certification.
From cerbaresearch.com
SRL Laboratories CAP certification Cerba Research Cap Iso Certification Things to know for cap laboratory accreditation. Accreditation is a continual process. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. The cap’s iso 15189 accreditation program. By the end of this period,. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Ten steps to cap laboratory accreditation. Learn more about our global. Cap Iso Certification.
From asia-bolt.com
Bolts and Nuts Asia Bolts Industries LLC fasteners manufacturer dubai Cap Iso Certification How long has the cap been accrediting to iso 15189? • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Accreditation is a continual process. College of american pathologists (cap) ngsp certificate of traceability. Things to know for cap laboratory accreditation. By the end of this period,. The. Cap Iso Certification.
From www.alamy.com
iso certification banner template. iso certification ribbon label sign Cap Iso Certification College of american pathologists (cap) ngsp certificate of traceability. How long has the cap been accrediting to iso 15189? This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Things to know for cap laboratory. Cap Iso Certification.
From www.vecteezy.com
Iso certification . iso 90012015 logo . iso 9000 certification Premium Cap Iso Certification Why did the cap launch the accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. By the end of this period,. Ten steps to cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. The cap. Cap Iso Certification.
From isocouncil.com.au
Types of ISO certification THE ISO COUNCIL Cap Iso Certification The cap’s iso 15189 accreditation program. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Accreditation is a continual process. Things to know for cap laboratory accreditation. By the end of this period,. Why did the cap launch the accreditation. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. This program delivers the. Cap Iso Certification.
From www.freepik.com
Premium Vector ISO 9001 2015 Certification ISO 90012015 Logo ISO 9000 Cap Iso Certification Why did the cap launch the accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. By the end of this period,. The cap’s iso 15189 accreditation program. How long has the cap been accrediting to iso 15189? • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Things to know for cap laboratory accreditation. The cap. Cap Iso Certification.
From www.connectixcablingsystems.ie
ISO Certification CCS Cap Iso Certification Accreditation is a continual process. Things to know for cap laboratory accreditation. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. By the end of this period,. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard.. Cap Iso Certification.
From www.iso-certification.us
ISO Certification FAQ ISOCERTIFICATION.US Cap Iso Certification The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. How long has the cap been accrediting to iso 15189? This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Things to know for cap laboratory accreditation. Learn more about our global network of fully. Cap Iso Certification.
From www.tech-ceram.com
News TechCeram Cap Iso Certification The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189 standard. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. College of american pathologists (cap) ngsp certificate of traceability. Things to know. Cap Iso Certification.
From medical-x.com
We are ISO 90012015 certified! MedicalX Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Ten steps to cap laboratory accreditation. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. How long has the cap been accrediting to iso 15189? By the end of this period,. Why did the cap launch the accreditation. College of american pathologists (cap) ngsp certificate of traceability.. Cap Iso Certification.
From registrationshops.in
ISO Certification Cap Iso Certification This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Things to know for cap laboratory accreditation. The cap’s iso 15189 accreditation program. Why did the cap launch the accreditation. Ten steps to cap laboratory accreditation. The cap 15189 accreditation program offers you a personalized, flexible process for initial accreditation to the iso 15189. Cap Iso Certification.
From www.freepik.com
Premium Vector Set of iso certification stamp and labels quality Cap Iso Certification This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Accreditation is a continual process. Things to know for cap laboratory accreditation. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. The cap’s iso 15189 accreditation program. Why did the cap launch the accreditation. Ten steps to cap laboratory accreditation. College of american pathologists (cap) ngsp certificate. Cap Iso Certification.
From www.startupguruz.com
ISO Certification Registration Types Benefits Documents Procedure Cap Iso Certification • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. Ten steps to cap laboratory accreditation. College of american pathologists (cap) ngsp certificate of traceability. By the end of this period,. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide. Cap Iso Certification.
From www.freepik.com
Premium Vector ISO 9001 2015 Certification ISO 90012015 Logo ISO 9000 Cap Iso Certification College of american pathologists (cap) ngsp certificate of traceability. This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. • capは臨床検査成績評価プログラム(以下 capサーベイ)及び臨床検査室認定プログラ ム(以下lap)を実施 • capサーベイは、capに. Accreditation is a continual process. Things to know for cap laboratory accreditation. The cap’s iso 15189 accreditation program. Why did the cap launch the accreditation. By the end of. Cap Iso Certification.
From www.exportersindia.com
16 Amp cover / Cap, Certification ISO9001 2008 Certified, Color Cap Iso Certification This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. By the end of this period,. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality data and services across. College of american pathologists (cap) ngsp certificate of traceability. The cap 15189 accreditation program offers. Cap Iso Certification.
From www.certbodies.co.uk
Use of ISO Certification Marks & Logos Certification Bodies Cap Iso Certification How long has the cap been accrediting to iso 15189? This program delivers the prescriptive technical requirements established in the clia requirements with the core quality. College of american pathologists (cap) ngsp certificate of traceability. The cap’s iso 15189 accreditation program. Learn more about our global network of fully cap accredited, iso 15189 central labs that can provide the quality. Cap Iso Certification.