The Mole And Percentage Composition Review Answer Key . The volume occupied by 1 mole of gas at standard temperature and pressure. 10.10 percent composition review questions 1. What is the formula for calculating percent composition? Imagine that you have 1 mole of. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. The mole review 1) define “mole”. Mole calculations & percent composition. Atoms combined make up molecules, which are the measurement of. The conditions under which the volume of a gas is usually. 3) how many moles are present in 2.45 x 1023. If its molecular mass is 134 g/mol,. You will need a periodic table to look up molecular weights. The atomic mass is the amount of protons plus number of atoms. 2) how many moles are present in 34 grams of cu(oh) 2? Percentage of the total mass of a compound contributed by an element is the percentage of that element in the.
from studylib.net
3) how many moles are present in 2.45 x 1023. Mole calculations & percent composition. 10.10 percent composition review questions 1. If its molecular mass is 134 g/mol,. Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. You will need a periodic table to look up molecular weights. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. What information do you need. The atomic mass is the amount of protons plus number of atoms. Imagine that you have 1 mole of.
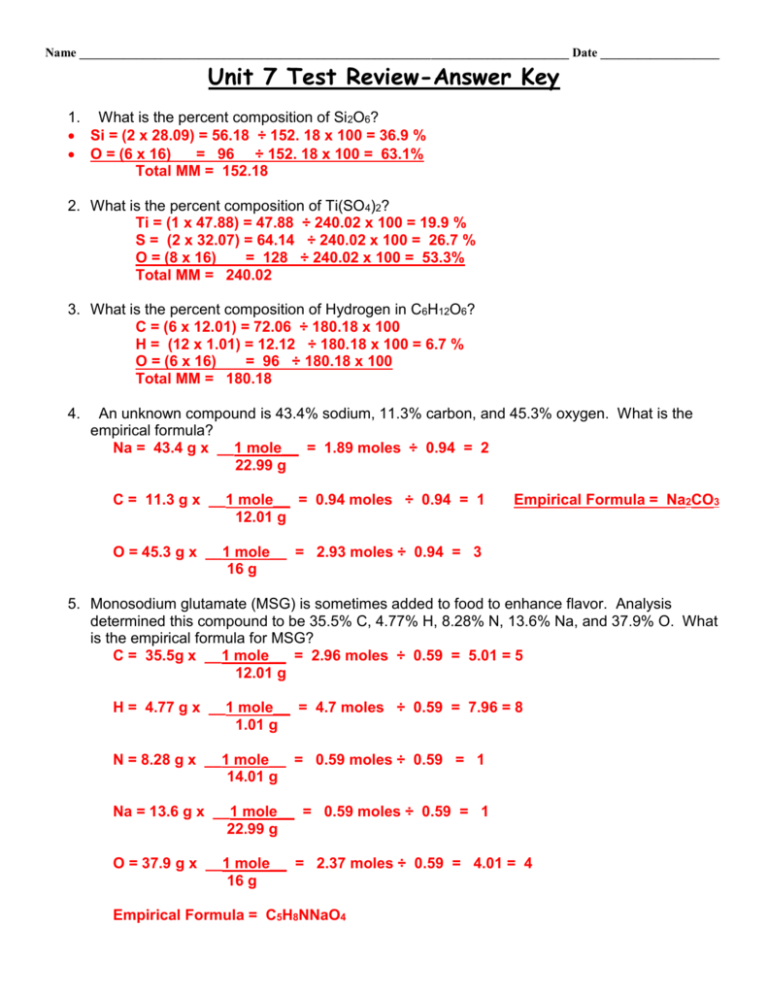
Unit 8 Mole Test Review AK
The Mole And Percentage Composition Review Answer Key 2) how many moles are present in 34 grams of cu(oh) 2? The conditions under which the volume of a gas is usually. You will need a periodic table to look up molecular weights. 10.10 percent composition review questions 1. Mole calculations & percent composition. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. The atomic mass is the amount of protons plus number of atoms. 2) how many moles are present in 34 grams of cu(oh) 2? If its molecular mass is 134 g/mol,. Atoms combined make up molecules, which are the measurement of. The volume occupied by 1 mole of gas at standard temperature and pressure. The mole review 1) define “mole”. What is the formula for calculating percent composition? What information do you need. Imagine that you have 1 mole of. 3) how many moles are present in 2.45 x 1023.
From dokumen.tips
(PDF) Chemical Composition Review Mole Calculations Percent The Mole And Percentage Composition Review Answer Key A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. What information do you need. 10.10 percent composition review questions 1. Mole calculations & percent composition. Atoms combined make up molecules, which are the measurement of. If its molecular mass is 134 g/mol,. Imagine that you have 1 mole of. The atomic mass is the amount of protons. The Mole And Percentage Composition Review Answer Key.
From quizzlibrarylane88.z13.web.core.windows.net
Mole Conversion Practice Problems Worksheet With Answers The Mole And Percentage Composition Review Answer Key The atomic mass is the amount of protons plus number of atoms. The mole review 1) define “mole”. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. What is the formula for calculating percent composition? 2) how many moles are present in 34 grams of cu(oh) 2? Mole calculations & percent composition. You will need a periodic. The Mole And Percentage Composition Review Answer Key.
From www.englishworksheet.my.id
Stoichiometry Worksheet Answer Key English Worksheet The Mole And Percentage Composition Review Answer Key Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. What information do you need. 2) how many moles are present in 34 grams of cu(oh) 2? Mole calculations & percent composition. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. The volume occupied by 1 mole. The Mole And Percentage Composition Review Answer Key.
From askfilo.com
The percentage composition of carbon by mole in methane is (2019 Main, 8 The Mole And Percentage Composition Review Answer Key 10.10 percent composition review questions 1. 2) how many moles are present in 34 grams of cu(oh) 2? What is the formula for calculating percent composition? Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. Mole calculations & percent composition. The conditions under which the volume of a gas. The Mole And Percentage Composition Review Answer Key.
From www.housview.com
Mole Conversions And Percent Composition Worksheet Answer Key Free The Mole And Percentage Composition Review Answer Key A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. You will need a periodic table to look up molecular weights. 10.10 percent composition review questions 1. Imagine that you have 1 mole of. What is the formula for calculating percent composition? 2) how many moles are present in 34 grams of cu(oh) 2? The mole review 1). The Mole And Percentage Composition Review Answer Key.
From worksheets.decoomo.com
30++ Mole Conversion Worksheet Answer Key With Work Worksheets Decoomo The Mole And Percentage Composition Review Answer Key Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. The volume occupied by 1 mole of gas at standard temperature and pressure. What is the formula for calculating percent composition? The mole review 1) define “mole”. Atoms combined make up molecules, which are the measurement of. 2) how many. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Molar Mass and Percent Composition The Mole And Percentage Composition Review Answer Key If its molecular mass is 134 g/mol,. Imagine that you have 1 mole of. The mole review 1) define “mole”. What information do you need. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. What is the formula for calculating percent composition? 3) how many moles are present in 2.45 x 1023. You will need a periodic. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Worksheet Mole Calculations & Percent Composition The Mole And Percentage Composition Review Answer Key The volume occupied by 1 mole of gas at standard temperature and pressure. You will need a periodic table to look up molecular weights. The atomic mass is the amount of protons plus number of atoms. 2) how many moles are present in 34 grams of cu(oh) 2? The mole review 1) define “mole”. Atoms combined make up molecules, which. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Mole and Percentage Composition Practice Problems The Mole And Percentage Composition Review Answer Key The volume occupied by 1 mole of gas at standard temperature and pressure. 2) how many moles are present in 34 grams of cu(oh) 2? Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. 10.10 percent composition review questions 1. Imagine that you have 1 mole of. What information. The Mole And Percentage Composition Review Answer Key.
From www.lessonplanet.com
Molar Mass and Percentage Composition Worksheet for 9th 12th Grade The Mole And Percentage Composition Review Answer Key The volume occupied by 1 mole of gas at standard temperature and pressure. Atoms combined make up molecules, which are the measurement of. What information do you need. 3) how many moles are present in 2.45 x 1023. Mole calculations & percent composition. If its molecular mass is 134 g/mol,. 2) how many moles are present in 34 grams of. The Mole And Percentage Composition Review Answer Key.
From www.scribd.com
Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key The Mole And Percentage Composition Review Answer Key The conditions under which the volume of a gas is usually. 2) how many moles are present in 34 grams of cu(oh) 2? Imagine that you have 1 mole of. If its molecular mass is 134 g/mol,. 10.10 percent composition review questions 1. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. You will need a periodic. The Mole And Percentage Composition Review Answer Key.
From rajuaji1.blogspot.com
Mole Calculation Practice Worksheet Answers / Chemistry Mole Concept The Mole And Percentage Composition Review Answer Key The mole review 1) define “mole”. 3) how many moles are present in 2.45 x 1023. You will need a periodic table to look up molecular weights. What information do you need. The volume occupied by 1 mole of gas at standard temperature and pressure. If its molecular mass is 134 g/mol,. Atoms combined make up molecules, which are the. The Mole And Percentage Composition Review Answer Key.
From www.studypool.com
SOLUTION Chemistry formulas percentage composition and the mole The Mole And Percentage Composition Review Answer Key You will need a periodic table to look up molecular weights. The mole review 1) define “mole”. What information do you need. 3) how many moles are present in 2.45 x 1023. Imagine that you have 1 mole of. 10.10 percent composition review questions 1. Atoms combined make up molecules, which are the measurement of. Percentage of the total mass. The Mole And Percentage Composition Review Answer Key.
From www.unmisravle.com
Percent Composition And Molecular Formula Worksheet Key Worksheets The Mole And Percentage Composition Review Answer Key The mole review 1) define “mole”. 10.10 percent composition review questions 1. Imagine that you have 1 mole of. You will need a periodic table to look up molecular weights. What information do you need. The atomic mass is the amount of protons plus number of atoms. 2) how many moles are present in 34 grams of cu(oh) 2? A. The Mole And Percentage Composition Review Answer Key.
From www.slideserve.com
PPT Percent Composition, Empirical Formulas, Molecular Formulas The Mole And Percentage Composition Review Answer Key The atomic mass is the amount of protons plus number of atoms. 10.10 percent composition review questions 1. Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. The volume occupied by 1 mole of gas at standard temperature and pressure. If its molecular mass is 134 g/mol,. You will. The Mole And Percentage Composition Review Answer Key.
From www.studypool.com
SOLUTION Chemistry formulas percentage composition and the mole The Mole And Percentage Composition Review Answer Key You will need a periodic table to look up molecular weights. What is the formula for calculating percent composition? If its molecular mass is 134 g/mol,. Atoms combined make up molecules, which are the measurement of. Mole calculations & percent composition. The volume occupied by 1 mole of gas at standard temperature and pressure. 10.10 percent composition review questions 1.. The Mole And Percentage Composition Review Answer Key.
From www.pinterest.ca
Mole Conversions Worksheets Answer Key Chemistry worksheets, Mole The Mole And Percentage Composition Review Answer Key What information do you need. Mole calculations & percent composition. 2) how many moles are present in 34 grams of cu(oh) 2? 3) how many moles are present in 2.45 x 1023. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. Atoms combined make up molecules, which are the measurement of. What is the formula for calculating. The Mole And Percentage Composition Review Answer Key.
From www.liveworksheets.com
Chemistry Mole Calculation Worksheet worksheet The Mole And Percentage Composition Review Answer Key What information do you need. The volume occupied by 1 mole of gas at standard temperature and pressure. 3) how many moles are present in 2.45 x 1023. If its molecular mass is 134 g/mol,. The mole review 1) define “mole”. You will need a periodic table to look up molecular weights. The conditions under which the volume of a. The Mole And Percentage Composition Review Answer Key.
From answermagicrichter.z19.web.core.windows.net
Percent Composition Worksheet 2 Answer Key The Mole And Percentage Composition Review Answer Key Imagine that you have 1 mole of. You will need a periodic table to look up molecular weights. What is the formula for calculating percent composition? What information do you need. Atoms combined make up molecules, which are the measurement of. Percentage of the total mass of a compound contributed by an element is the percentage of that element in. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Unit 8 Mole Test Review AK The Mole And Percentage Composition Review Answer Key Imagine that you have 1 mole of. What is the formula for calculating percent composition? Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. The volume occupied by 1 mole of gas at standard temperature and pressure. 3) how many moles are present in 2.45 x 1023. 10.10 percent. The Mole And Percentage Composition Review Answer Key.
From printablemediakillian.z19.web.core.windows.net
Percentage Composition Worksheet Answer Key The Mole And Percentage Composition Review Answer Key The conditions under which the volume of a gas is usually. What information do you need. Atoms combined make up molecules, which are the measurement of. The volume occupied by 1 mole of gas at standard temperature and pressure. Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. 2). The Mole And Percentage Composition Review Answer Key.
From www.youtube.com
Chemcial Formulas Part 1 Mole Review YouTube The Mole And Percentage Composition Review Answer Key What is the formula for calculating percent composition? What information do you need. Imagine that you have 1 mole of. Atoms combined make up molecules, which are the measurement of. 10.10 percent composition review questions 1. 3) how many moles are present in 2.45 x 1023. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. Mole calculations. The Mole And Percentage Composition Review Answer Key.
From www.housview.com
Mole Conversions And Percent Composition Worksheet Answers Free The Mole And Percentage Composition Review Answer Key Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. Atoms combined make up molecules, which are the measurement of. What is the formula for calculating percent composition? The conditions under which the volume of a gas is usually. Imagine that you have 1 mole of. The mole review 1). The Mole And Percentage Composition Review Answer Key.
From www.pinterest.com
Help your students review Stoichiometry and The Mole using this huge The Mole And Percentage Composition Review Answer Key The volume occupied by 1 mole of gas at standard temperature and pressure. The conditions under which the volume of a gas is usually. 10.10 percent composition review questions 1. What is the formula for calculating percent composition? The mole review 1) define “mole”. Imagine that you have 1 mole of. Mole calculations & percent composition. If its molecular mass. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Mole Review Problems (Be sure to SHOW ALL WORK and circle The Mole And Percentage Composition Review Answer Key A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. The atomic mass is the amount of protons plus number of atoms. Mole calculations & percent composition. What is the formula for calculating percent composition? If its molecular mass is 134 g/mol,. What information do you need. Atoms combined make up molecules, which are the measurement of. Imagine. The Mole And Percentage Composition Review Answer Key.
From classzonecombatant.z14.web.core.windows.net
Percent Composition Examples With Answers The Mole And Percentage Composition Review Answer Key 2) how many moles are present in 34 grams of cu(oh) 2? 3) how many moles are present in 2.45 x 1023. Mole calculations & percent composition. The atomic mass is the amount of protons plus number of atoms. Imagine that you have 1 mole of. If its molecular mass is 134 g/mol,. The mole review 1) define “mole”. 10.10. The Mole And Percentage Composition Review Answer Key.
From studylib.net
Empirical and Molecular Formulas Worksheet 1 1. The percentage The Mole And Percentage Composition Review Answer Key Mole calculations & percent composition. You will need a periodic table to look up molecular weights. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. 3) how many moles are present in 2.45 x 1023. The volume occupied by 1 mole of gas at standard temperature and pressure. 10.10 percent composition review questions 1. Imagine that you. The Mole And Percentage Composition Review Answer Key.
From www.scribd.com
Limiting Reagents and Percentage Yield Worksheet answers.doc Mole The Mole And Percentage Composition Review Answer Key If its molecular mass is 134 g/mol,. You will need a periodic table to look up molecular weights. 10.10 percent composition review questions 1. The atomic mass is the amount of protons plus number of atoms. Atoms combined make up molecules, which are the measurement of. The volume occupied by 1 mole of gas at standard temperature and pressure. What. The Mole And Percentage Composition Review Answer Key.
From www.worksheeto.com
8 Best Images of Percent Composition Worksheet Answer Key Percent The Mole And Percentage Composition Review Answer Key You will need a periodic table to look up molecular weights. What is the formula for calculating percent composition? Atoms combined make up molecules, which are the measurement of. The mole review 1) define “mole”. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. Imagine that you have 1 mole of. What information do you need. Percentage. The Mole And Percentage Composition Review Answer Key.
From askfilo.com
MOLE CONCEPT DPP4 [Percentage composition, Emperical formula, Molecular The Mole And Percentage Composition Review Answer Key 2) how many moles are present in 34 grams of cu(oh) 2? Atoms combined make up molecules, which are the measurement of. If its molecular mass is 134 g/mol,. The conditions under which the volume of a gas is usually. You will need a periodic table to look up molecular weights. What information do you need. Mole calculations & percent. The Mole And Percentage Composition Review Answer Key.
From www.housview.com
Percentage Composition Worksheets Answer Key The Mole And Percentage Composition Review Answer Key Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. Mole calculations & percent composition. The atomic mass is the amount of protons plus number of atoms. 3) how many moles are present in 2.45 x 1023. If. The Mole And Percentage Composition Review Answer Key.
From www.studypool.com
SOLUTION The mole and percentage composition Studypool The Mole And Percentage Composition Review Answer Key Imagine that you have 1 mole of. 10.10 percent composition review questions 1. The atomic mass is the amount of protons plus number of atoms. Mole calculations & percent composition. The mole review 1) define “mole”. Atoms combined make up molecules, which are the measurement of. You will need a periodic table to look up molecular weights. Percentage of the. The Mole And Percentage Composition Review Answer Key.
From www.worksheeto.com
8 Best Images of Percent Composition Worksheet Answer Key Percent The Mole And Percentage Composition Review Answer Key What is the formula for calculating percent composition? 2) how many moles are present in 34 grams of cu(oh) 2? Percentage of the total mass of a compound contributed by an element is the percentage of that element in the. Mole calculations & percent composition. Atoms combined make up molecules, which are the measurement of. 3) how many moles are. The Mole And Percentage Composition Review Answer Key.
From www.lessonplanet.com
Percentage Composition and Empirical & Molecular Formula Worksheet for The Mole And Percentage Composition Review Answer Key A compound is composed of 34.2% sodium, 17.7% carbon, and 47.6% oxygen. The atomic mass is the amount of protons plus number of atoms. Atoms combined make up molecules, which are the measurement of. What is the formula for calculating percent composition? Percentage of the total mass of a compound contributed by an element is the percentage of that element. The Mole And Percentage Composition Review Answer Key.
From byjus.com
Calculate the mole percentage of methanol and water in 60 The Mole And Percentage Composition Review Answer Key What is the formula for calculating percent composition? The conditions under which the volume of a gas is usually. 3) how many moles are present in 2.45 x 1023. The atomic mass is the amount of protons plus number of atoms. 10.10 percent composition review questions 1. Imagine that you have 1 mole of. Percentage of the total mass of. The Mole And Percentage Composition Review Answer Key.