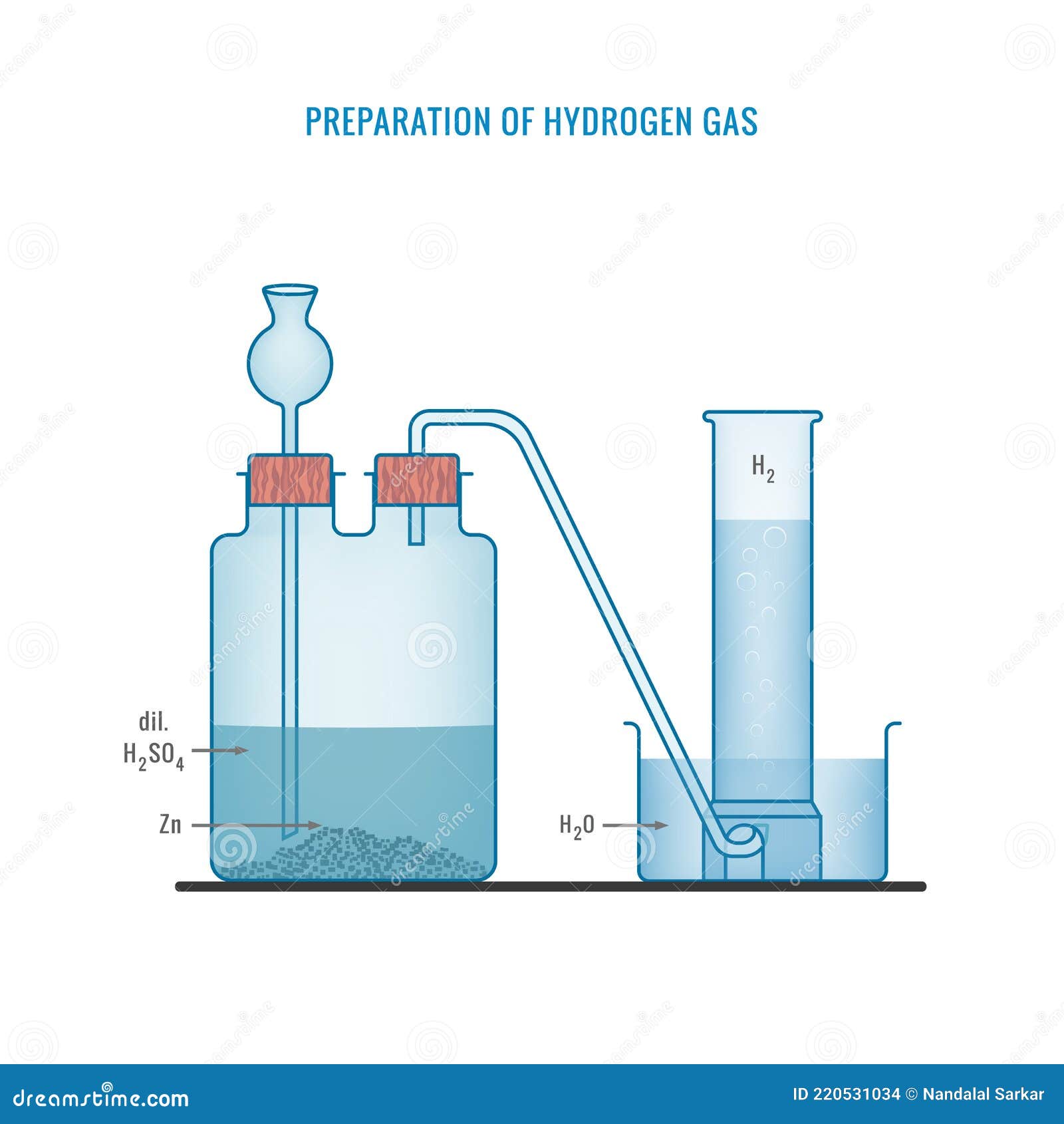
Zinc Chloride And Hydrogen Gas . In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). The chemical equation for this reaction is given by: The products of the reaction are aqueous zinc chloride and. Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Zn + 2hcl → zncl2 + h2. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. A.) write a balanced reaction. The hydrogen gas is seen in the form of. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. This reaction is highly exothermic, meaning it releases a When zinc is added to hydrochloric acid, a vigorous reaction takes place.
from www.dreamstime.com
Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The products of the reaction are aqueous zinc chloride and. The hydrogen gas is seen in the form of. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. This reaction is highly exothermic, meaning it releases a 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). Zn + 2hcl → zncl2 + h2. A.) write a balanced reaction. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride.
Preparation of Hydrogen Gas in Laboratory with the Help of Zinc and
Zinc Chloride And Hydrogen Gas Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. The hydrogen gas is seen in the form of. A.) write a balanced reaction. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. When zinc is added to hydrochloric acid, a vigorous reaction takes place. This reaction is highly exothermic, meaning it releases a You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. The products of the reaction are aqueous zinc chloride and. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). The chemical equation for this reaction is given by: Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. Zn + 2hcl → zncl2 + h2.
From www.pw.live
Zinc Chloride Formula Zinc Chloride And Hydrogen Gas The chemical equation for this reaction is given by: The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. A.) write a balanced reaction. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. Zn + 2hcl → zncl2 + h2. In order to obtain a hydrated form of the compound, hydrochloric. Zinc Chloride And Hydrogen Gas.
From www.alamy.com
Zinc reacting with hydrochloric acid. This reaction leads to the Zinc Chloride And Hydrogen Gas Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. A.) write a balanced reaction. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). In order to obtain a hydrated form of. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Zinc metal reacts with aqueous hydrochloric acid to produce a Zinc Chloride And Hydrogen Gas This reaction is highly exothermic, meaning it releases a Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The hydrogen gas is seen in the form of. The products of the reaction are aqueous zinc chloride and. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. When zinc is. Zinc Chloride And Hydrogen Gas.
From www.chegg.com
Solved In this problem, zinc reacts with hydrochloric acid Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. This reaction is highly exothermic, meaning it releases a The chemical equation for this reaction is given by: Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. Zn + 2hcl → zncl2 + h2. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to. Zinc Chloride And Hydrogen Gas.
From answerhappy.com
Metallic zinc reacts with hydrochloric acid (HCI) to form hydrogen gas Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. A.) write a balanced reaction. When zinc is added to hydrochloric acid, a vigorous reaction takes place. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. The chemical. Zinc Chloride And Hydrogen Gas.
From www.alamy.com
Zinc reacting with hydrochloric acid. This reaction leads to the Zinc Chloride And Hydrogen Gas The chemical equation for this reaction is given by: Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. The products of the reaction are aqueous zinc chloride and. A.) write a balanced reaction. When zinc is added to hydrochloric acid, a vigorous reaction takes place. The hydrogen gas is seen in the form of. The reaction. Zinc Chloride And Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Chloride And Hydrogen Gas A.) write a balanced reaction. The hydrogen gas is seen in the form of. This reaction is highly exothermic, meaning it releases a The products of the reaction are aqueous zinc chloride and. When zinc is added to hydrochloric acid, a vigorous reaction takes place. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc. Zinc Chloride And Hydrogen Gas.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Chloride And Hydrogen Gas The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The hydrogen gas is seen in the form of. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. The chemical equation for this reaction is. Zinc Chloride And Hydrogen Gas.
From brainly.in
Zinc sulphate reacts with hydrochloric acid and gives zinc chloride and Zinc Chloride And Hydrogen Gas The chemical equation for this reaction is given by: The products of the reaction are aqueous zinc chloride and. This reaction is highly exothermic, meaning it releases a In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. The hydrogen gas is seen in the form of. You're. Zinc Chloride And Hydrogen Gas.
From www.alamy.com
Labelled diagram for laboratory preparation of hydrogen from zinc and Zinc Chloride And Hydrogen Gas The chemical equation for this reaction is given by: You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). This reaction is highly exothermic, meaning it releases a The reaction between metallic zinc and hydrogen chloride. Zinc Chloride And Hydrogen Gas.
From www.chegg.com
Solved Aqueous zinc chloride and hydrogen gas are formed by Zinc Chloride And Hydrogen Gas Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. The reaction between metallic zinc and hydrogen chloride gas. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Zinc Chloride And Hydrogen Gas The hydrogen gas is seen in the form of. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). The products of the reaction are aqueous zinc chloride and. This reaction is highly exothermic, meaning it releases. Zinc Chloride And Hydrogen Gas.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. The hydrogen gas is seen in the form of. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to. Zinc Chloride And Hydrogen Gas.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it.. Zinc Chloride And Hydrogen Gas.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Chloride And Hydrogen Gas Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The products of the reaction are aqueous zinc chloride and. The chemical equation for this reaction is given by: Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen. Zinc Chloride And Hydrogen Gas.
From www.sciencephoto.com
Zinc & Hydrogen chloride Reacting Stock Image C027/7927 Science Zinc Chloride And Hydrogen Gas When zinc is added to hydrochloric acid, a vigorous reaction takes place. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The products of the reaction are aqueous zinc chloride and. A.) write a balanced reaction. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. You're dealing. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Zinc metal dissolves in hydrochloric acid to yield hydrogen gas Zinc Chloride And Hydrogen Gas A.) write a balanced reaction. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The products of the reaction are aqueous zinc chloride and. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. In order to obtain a hydrated form of the compound, hydrochloric acid can be. Zinc Chloride And Hydrogen Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric Acid Zinc Chloride And Hydrogen Gas Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The chemical equation for this reaction is given by: Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. You're dealing with a single replacement. Zinc Chloride And Hydrogen Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Hydrogen Gas Lab Zinc And Hydrochloric Acid Zinc Chloride And Hydrogen Gas The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The products of the reaction are aqueous zinc chloride and. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). The reaction between metallic. Zinc Chloride And Hydrogen Gas.
From hydrogengasgaosube.blogspot.com
Hydrogen Gas Zinc Chloride And Hydrogen Gas Zinc Chloride And Hydrogen Gas Zn + 2hcl → zncl2 + h2. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. This reaction is highly exothermic, meaning it releases a Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The reaction between metallic zinc and hydrogen chloride gas. Zinc Chloride And Hydrogen Gas.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The reaction between. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Part 3C Writing Balanced Chemical Equations Write a balanced Zinc Chloride And Hydrogen Gas In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. The chemical equation for this reaction is given by: Zinc metal reacts with hydrochloric acid to form zinc chloride. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED When zinc is dropped into hydrochloric acid, which gas is Zinc Chloride And Hydrogen Gas Zn + 2hcl → zncl2 + h2. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. When zinc is added to hydrochloric acid, a vigorous reaction takes place. Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. You're dealing with a single replacement. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVEDHydrogen gas can be prepared by reaction of zinc metal with Zinc Chloride And Hydrogen Gas The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Zn + 2hcl → zncl2 + h2. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The products of the reaction are aqueous zinc chloride and. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and. Zinc Chloride And Hydrogen Gas.
From brainly.in
zinc reacts with dilute hydrochloric acid to give zinc chloride and Zinc Chloride And Hydrogen Gas This reaction is highly exothermic, meaning it releases a The chemical equation for this reaction is given by: Zinc metal reacts with hydrochloric acid to form zinc chloride and hydrogen gas. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). Zinc is more reactive than hydrogen, it reacts with hydrochloric acid. Zinc Chloride And Hydrogen Gas.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Chloride And Hydrogen Gas Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. Zn + 2hcl → zncl2 + h2. When zinc is added to hydrochloric acid, a vigorous reaction takes place. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The chemical equation for this reaction is. Zinc Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid to produce zinc Zinc Chloride And Hydrogen Gas Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). When zinc is added to hydrochloric acid, a vigorous reaction takes place. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence. Zinc Chloride And Hydrogen Gas.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc Chloride And Hydrogen Gas The products of the reaction are aqueous zinc chloride and. Zn + 2hcl → zncl2 + h2. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. The hydrogen gas is seen in the form of. In order to. Zinc Chloride And Hydrogen Gas.
From www.alamy.com
Zinc reacting with hydrochloric acid. Zinc (Zn) reacts vigorously with Zinc Chloride And Hydrogen Gas Zn + 2hcl → zncl2 + h2. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. A.) write a balanced reaction. The products of the reaction are aqueous zinc chloride and. The hydrogen gas is seen in the form of. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas.. Zinc Chloride And Hydrogen Gas.
From www.dreamstime.com
Preparation of Hydrogen Gas in Laboratory with the Help of Zinc and Zinc Chloride And Hydrogen Gas You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. The chemical equation for this reaction is given by: A.) write a balanced reaction. Zn + 2hcl → zncl2 + h2. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. This reaction is highly exothermic, meaning it. Zinc Chloride And Hydrogen Gas.
From byjus.com
a Draw a labelled diagram for the laboratory preparation of hydrogen Zinc Chloride And Hydrogen Gas When zinc is added to hydrochloric acid, a vigorous reaction takes place. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. Zn + 2hcl → zncl2 + h2. The zinc begins to dissolve in the acid, producing hydrogen gas and forming zinc chloride. Zinc reacts with dilute hydrochloric acid to. Zinc Chloride And Hydrogen Gas.
From www.youtube.com
DIY Hydrogen Gas (Hydrochloric Acid + Zinc) YouTube Zinc Chloride And Hydrogen Gas The chemical equation for this reaction is given by: 1 when zinc (zn) reacts with hydrochloric acid (hcl), it forms zinc chloride (zncl2) and hydrogen gas (h2). When zinc is added to hydrochloric acid, a vigorous reaction takes place. The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. Zinc metal reacts with hydrochloric. Zinc Chloride And Hydrogen Gas.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Chloride And Hydrogen Gas This reaction is highly exothermic, meaning it releases a The chemical equation for this reaction is given by: The reaction between metallic zinc and hydrogen chloride gas yields an anhydrous form of zinc chloride. A.) write a balanced reaction. When zinc is added to hydrochloric acid, a vigorous reaction takes place. Zinc is more reactive than hydrogen, it reacts with. Zinc Chloride And Hydrogen Gas.
From www.gauthmath.com
Solved Zinc reacts with dilute hydrochloric acid to form zinc chloride Zinc Chloride And Hydrogen Gas Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. The products of the reaction are aqueous zinc chloride and. Zn + 2hcl → zncl2 + h2. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas and hence displace it. The chemical equation for this reaction is given by: Zinc metal. Zinc Chloride And Hydrogen Gas.
From www.gauthmath.com
Solved 1. Answer questions a and b. a. Zinc is reacted with hydrogen Zinc Chloride And Hydrogen Gas This reaction is highly exothermic, meaning it releases a Zn + 2hcl → zncl2 + h2. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas. You're dealing with a single replacement reaction in which zinc displaces the hydrogen from hydrochloric acid. Zinc is more reactive than hydrogen, it reacts with hydrochloric acid to release hydrogen gas. Zinc Chloride And Hydrogen Gas.