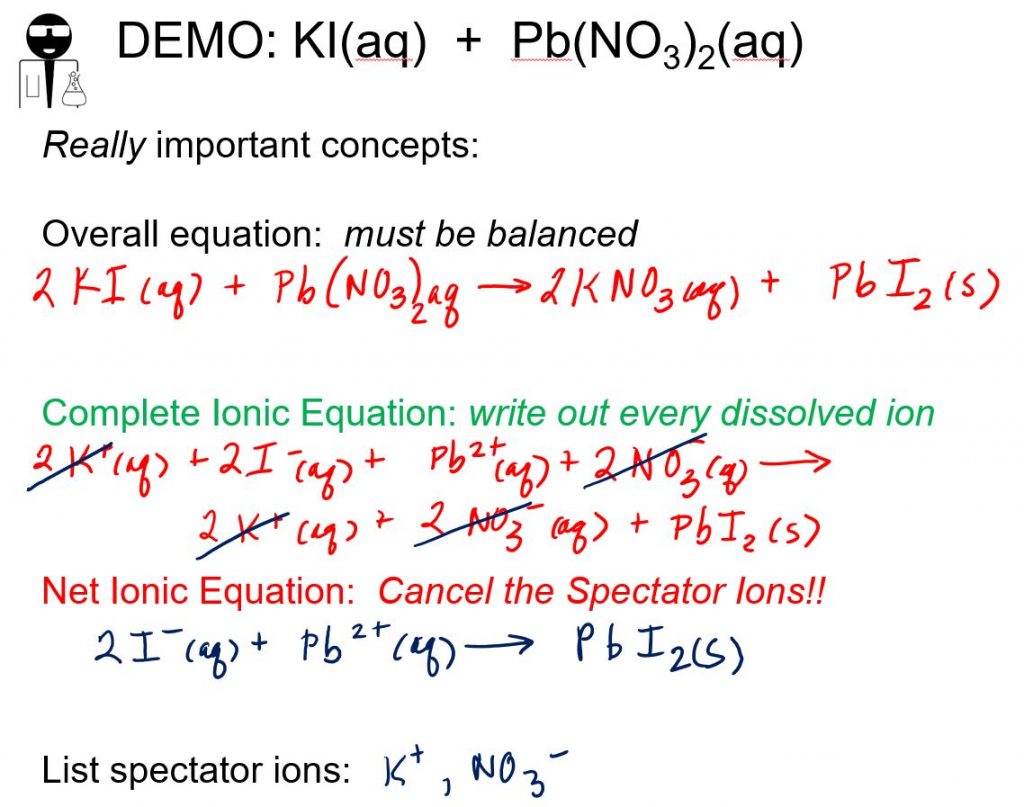
Lead Acetate And Sodium Chloride . lead forms two cations, pb2+ and pb4+. complete step by step answer: lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead belongs to the group. Lead chloride, lead bromide, lead iodide. Lead acetate was first produced in the united states in 1994. Because the solution also contains nh 4 + and i − ions, the possible products of. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Aqueous solutions of rubidium hydroxide and cobalt(ii). Both cations can form precipitates and solutions with anions and give colours. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. aqueous solutions of barium chloride and lithium sulfate are mixed.
from exoncvmev.blob.core.windows.net
Both cations can form precipitates and solutions with anions and give colours. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Aqueous solutions of rubidium hydroxide and cobalt(ii). watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Because the solution also contains nh 4 + and i − ions, the possible products of. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. complete step by step answer: Lead belongs to the group. aqueous solutions of barium chloride and lithium sulfate are mixed. lead forms two cations, pb2+ and pb4+.
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons blog
Lead Acetate And Sodium Chloride Because the solution also contains nh 4 + and i − ions, the possible products of. Because the solution also contains nh 4 + and i − ions, the possible products of. lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Aqueous solutions of rubidium hydroxide and cobalt(ii). lead forms two cations, pb2+ and pb4+. Lead acetate was first produced in the united states in 1994. Lead belongs to the group. aqueous solutions of barium chloride and lithium sulfate are mixed. complete step by step answer: Both cations can form precipitates and solutions with anions and give colours. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead chloride, lead bromide, lead iodide. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions react PowerPoint Lead Acetate And Sodium Chloride thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Lead acetate was first produced in the united states in 1994. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead chloride, lead bromide, lead iodide. lead(ii) chloride can. Lead Acetate And Sodium Chloride.
From www.numerade.com
SOLVED Consider the mechanism for the Nucleophilic Acyl Substitution reaction of acetyl Lead Acetate And Sodium Chloride complete step by step answer: Lead chloride, lead bromide, lead iodide. lead forms two cations, pb2+ and pb4+. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution.. Lead Acetate And Sodium Chloride.
From www.youtube.com
Sodium sulphate and Lead Acetate YouTube Lead Acetate And Sodium Chloride complete step by step answer: Lead chloride, lead bromide, lead iodide. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. soluble sulfates, including dilute sulfuric acid,. Lead Acetate And Sodium Chloride.
From sielc.com
Lead(IV) acetate SIELC Lead Acetate And Sodium Chloride soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead acetate was first produced in the united states in 1994. Lead chloride, lead bromide, lead iodide. Both cations can form precipitates and solutions with anions and give colours. complete step by step answer: thus solid lead acetate dissolves. Lead Acetate And Sodium Chloride.
From dxoqdjcqk.blob.core.windows.net
Lead Chloride Formula at Joesph Hernandez blog Lead Acetate And Sodium Chloride Aqueous solutions of rubidium hydroxide and cobalt(ii). Because the solution also contains nh 4 + and i − ions, the possible products of. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble. Lead Acetate And Sodium Chloride.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Lead Acetate And Sodium Chloride Lead acetate was first produced in the united states in 1994. Aqueous solutions of rubidium hydroxide and cobalt(ii). thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. lead forms two cations, pb2+ and pb4+. Both cations can form precipitates and solutions with anions and give colours. complete. Lead Acetate And Sodium Chloride.
From dxovcakol.blob.core.windows.net
If You Dissolve Lead Ii Nitrate And Potassium Iodide at Carmen Acker blog Lead Acetate And Sodium Chloride complete step by step answer: Both cations can form precipitates and solutions with anions and give colours. aqueous solutions of barium chloride and lithium sulfate are mixed. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: lead forms two cations, pb2+ and pb4+. watch this video. Lead Acetate And Sodium Chloride.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons blog Lead Acetate And Sodium Chloride aqueous solutions of barium chloride and lithium sulfate are mixed. Lead belongs to the group. lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Because the solution. Lead Acetate And Sodium Chloride.
From www.numerade.com
SOLVED ILead(II) acetate and sodium carbonate (3Lead(II) acetate and sodium chloride I4Lead Lead Acetate And Sodium Chloride complete step by step answer: Aqueous solutions of rubidium hydroxide and cobalt(ii). thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Lead acetate was first produced in the united states in 1994. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble. Lead Acetate And Sodium Chloride.
From www.youtube.com
9. Lead Tetra acetate YouTube Lead Acetate And Sodium Chloride Lead acetate was first produced in the united states in 1994. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. lead forms two cations, pb2+ and pb4+. Because the solution also contains nh 4 + and i − ions, the possible products of. Both cations can form. Lead Acetate And Sodium Chloride.
From www.labdepotinc.com
Lead Acetate, Trihydrate, Crystal, Reagent, ACS Lead Acetate And Sodium Chloride soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. Both cations can form precipitates and solutions with anions and give colours. Aqueous solutions of rubidium hydroxide and cobalt(ii). Lead. Lead Acetate And Sodium Chloride.
From www.chegg.com
Part A sodium chloride and lead(II) acetate Express Lead Acetate And Sodium Chloride lead forms two cations, pb2+ and pb4+. Both cations can form precipitates and solutions with anions and give colours. Lead acetate was first produced in the united states in 1994. Because the solution also contains nh 4 + and i − ions, the possible products of. Aqueous solutions of rubidium hydroxide and cobalt(ii). soluble sulfates, including dilute sulfuric. Lead Acetate And Sodium Chloride.
From testbook.com
Lead (IV) Acetate Formula, Properties, Structure, and Uses Lead Acetate And Sodium Chloride Lead chloride, lead bromide, lead iodide. Both cations can form precipitates and solutions with anions and give colours. lead forms two cations, pb2+ and pb4+. Lead acetate was first produced in the united states in 1994. Because the solution also contains nh 4 + and i − ions, the possible products of. lead(ii) chloride can be made as. Lead Acetate And Sodium Chloride.
From www.fishersci.com
Lead Acetate, 5 (w/v) Solution, Spectrum compounds Fisher Scientific Lead Acetate And Sodium Chloride watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Aqueous solutions of rubidium hydroxide and cobalt(ii). lead forms two cations, pb2+ and pb4+. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Lead belongs to the. Lead Acetate And Sodium Chloride.
From www.pw.live
Lead Acetate Formula, Structure, Molecular Weight, And Molar Mass Lead Acetate And Sodium Chloride Aqueous solutions of rubidium hydroxide and cobalt(ii). watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead belongs to the group. Lead acetate was first produced in the united. Lead Acetate And Sodium Chloride.
From www.youtube.com
Copper(II) acetate, Lead(II) acetate and Lead(II) chloride preparation YouTube Lead Acetate And Sodium Chloride watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. complete step by step answer: Lead belongs to the group. aqueous solutions of barium chloride and lithium sulfate are mixed. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than. Lead Acetate And Sodium Chloride.
From www.carolina.com
Lead Acetate Trihydrate, Laboratory Grade, 500 g Lead Acetate And Sodium Chloride Aqueous solutions of rubidium hydroxide and cobalt(ii). soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead chloride, lead bromide, lead iodide. complete step by step answer: Lead acetate was first produced in the united states in 1994. lead(ii) chloride can be made as a white precipitate by. Lead Acetate And Sodium Chloride.
From www.sciencecompany.com
A.C.S. Grade Lead Acetate Trihydrate, 100g for sale. Buy from The Science Company. Lead Acetate And Sodium Chloride complete step by step answer: aqueous solutions of barium chloride and lithium sulfate are mixed. Aqueous solutions of rubidium hydroxide and cobalt(ii). soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Both cations can form precipitates and solutions with anions and give colours. thus solid lead acetate. Lead Acetate And Sodium Chloride.
From www.youtube.com
Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic Lead Acetate And Sodium Chloride Lead belongs to the group. Aqueous solutions of rubidium hydroxide and cobalt(ii). Because the solution also contains nh 4 + and i − ions, the possible products of. complete step by step answer: Both cations can form precipitates and solutions with anions and give colours. aqueous solutions of barium chloride and lithium sulfate are mixed. Lead chloride, lead. Lead Acetate And Sodium Chloride.
From www.youtube.com
Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic Lead Acetate And Sodium Chloride aqueous solutions of barium chloride and lithium sulfate are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Lead belongs to the group. Lead acetate was first produced in the united states in 1994. Both cations can form precipitates and solutions with anions and give colours.. Lead Acetate And Sodium Chloride.
From www.youtube.com
Double displacement NaCl + Pb(NO3)2 Sodium chloride + Lead(ii) nitrate Precipitation Lead Acetate And Sodium Chloride lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. lead forms two cations, pb2+ and pb4+. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Because the solution also contains nh 4 + and i − ions,. Lead Acetate And Sodium Chloride.
From www.teachoo.com
In the double displacement reaction b/w aqueo (MCQ) Class 10 Science Lead Acetate And Sodium Chloride Aqueous solutions of rubidium hydroxide and cobalt(ii). complete step by step answer: Both cations can form precipitates and solutions with anions and give colours. Lead chloride, lead bromide, lead iodide. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead belongs to the group. watch this video to. Lead Acetate And Sodium Chloride.
From www.numerade.com
SOLVEDDoes a reaction occur when aqueous solutions of sodium sulfate and lead(II) nitrate are Lead Acetate And Sodium Chloride Both cations can form precipitates and solutions with anions and give colours. lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. Lead acetate was first produced in the united states in 1994. thus solid lead acetate dissolves in water to give pb 2 + and ch 3. Lead Acetate And Sodium Chloride.
From www.numerade.com
SOLVEDEnter a molecular equation for the precipitation reaction that occurs (if any) when each Lead Acetate And Sodium Chloride Because the solution also contains nh 4 + and i − ions, the possible products of. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Aqueous solutions of rubidium hydroxide and cobalt(ii). soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than. Lead Acetate And Sodium Chloride.
From haihangindustry.en.made-in-china.com
China Lead Acetate Trihydrate CAS 6080564 China Lead Acetate Trihydrate, 6080564 Lead Acetate And Sodium Chloride aqueous solutions of barium chloride and lithium sulfate are mixed. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. lead(ii) chloride can be made as a. Lead Acetate And Sodium Chloride.
From www.numerade.com
SOLVED Double replacements Aluminum bromide and Iithium hydroxide Zinc chloride and ammonium Lead Acetate And Sodium Chloride soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: Lead belongs to the group. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. complete step by step answer: Lead chloride, lead bromide, lead iodide. aqueous solutions. Lead Acetate And Sodium Chloride.
From exoncvmev.blob.core.windows.net
Lead Ii Nitrate And Sodium Chloride Balanced Equation at Janice Timmons blog Lead Acetate And Sodium Chloride Lead chloride, lead bromide, lead iodide. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. Lead acetate was first produced in the united states in 1994. Both cations can form precipitates and solutions with anions and give colours. Because the solution also contains nh 4 + and i −. Lead Acetate And Sodium Chloride.
From martlabpro.com
Understanding Lead Acetate Molar Mass In 2023 MartLabPro Lead Acetate And Sodium Chloride watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Lead acetate was first produced in the united states in 1994. Because the solution also contains nh 4 + and i − ions, the possible products of. Both cations can form precipitates and solutions with anions and give colours.. Lead Acetate And Sodium Chloride.
From www.chegg.com
Solved sodium chloride and lead(II) acetate potassium Lead Acetate And Sodium Chloride Both cations can form precipitates and solutions with anions and give colours. Because the solution also contains nh 4 + and i − ions, the possible products of. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. lead forms two cations, pb2+ and pb4+. thus solid. Lead Acetate And Sodium Chloride.
From thaipurescience.com
Lead(II) acetate trihydrate Thai Pure Science Lead Acetate And Sodium Chloride complete step by step answer: Both cations can form precipitates and solutions with anions and give colours. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: lead forms two cations, pb2+ and pb4+. Lead acetate was first produced in the united states in 1994. aqueous solutions of. Lead Acetate And Sodium Chloride.
From www.ceramic-glazes.com
Lead Acetate Lead(II) acetate trihydrate chemical compound Lead Acetate And Sodium Chloride Lead belongs to the group. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. Lead chloride, lead bromide, lead iodide. Because the solution also contains nh 4 + and i − ions, the possible products of. Both cations can form precipitates and solutions with anions and give colours.. Lead Acetate And Sodium Chloride.
From www.chegg.com
Solved Part A sodium chloride and lead (II) acetate Express Lead Acetate And Sodium Chloride lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. Lead acetate was first produced in the united states in 1994. aqueous solutions of barium chloride and lithium sulfate are mixed. Lead chloride, lead bromide, lead iodide. Lead belongs to the group. Both cations can form precipitates and. Lead Acetate And Sodium Chloride.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry PowerPoint Presentation ID6200909 Lead Acetate And Sodium Chloride aqueous solutions of barium chloride and lithium sulfate are mixed. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is much less soluble than lead chloride: lead forms two cations, pb2+ and pb4+. Both cations can form precipitates and solutions with anions and give colours. Lead acetate was first produced in the united states in. Lead Acetate And Sodium Chloride.
From excichem.com
51404694LeadAcetateBasic500 Excichem Research Chemicals for Laboratories. Lead Acetate And Sodium Chloride Lead belongs to the group. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. watch this video to learn about precipitation reactions, a type of chemical reaction that produces a solid from two aqueous. aqueous solutions of barium chloride and lithium sulfate are mixed. Lead chloride, lead. Lead Acetate And Sodium Chloride.
From www.youtube.com
How to Write the Formula for Lead (II) acetate YouTube Lead Acetate And Sodium Chloride complete step by step answer: lead(ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead(ii) nitrate solution. thus solid lead acetate dissolves in water to give pb 2 + and ch 3 co 2 − ions. soluble sulfates, including dilute sulfuric acid, precipitate white lead sulfate, which is. Lead Acetate And Sodium Chloride.