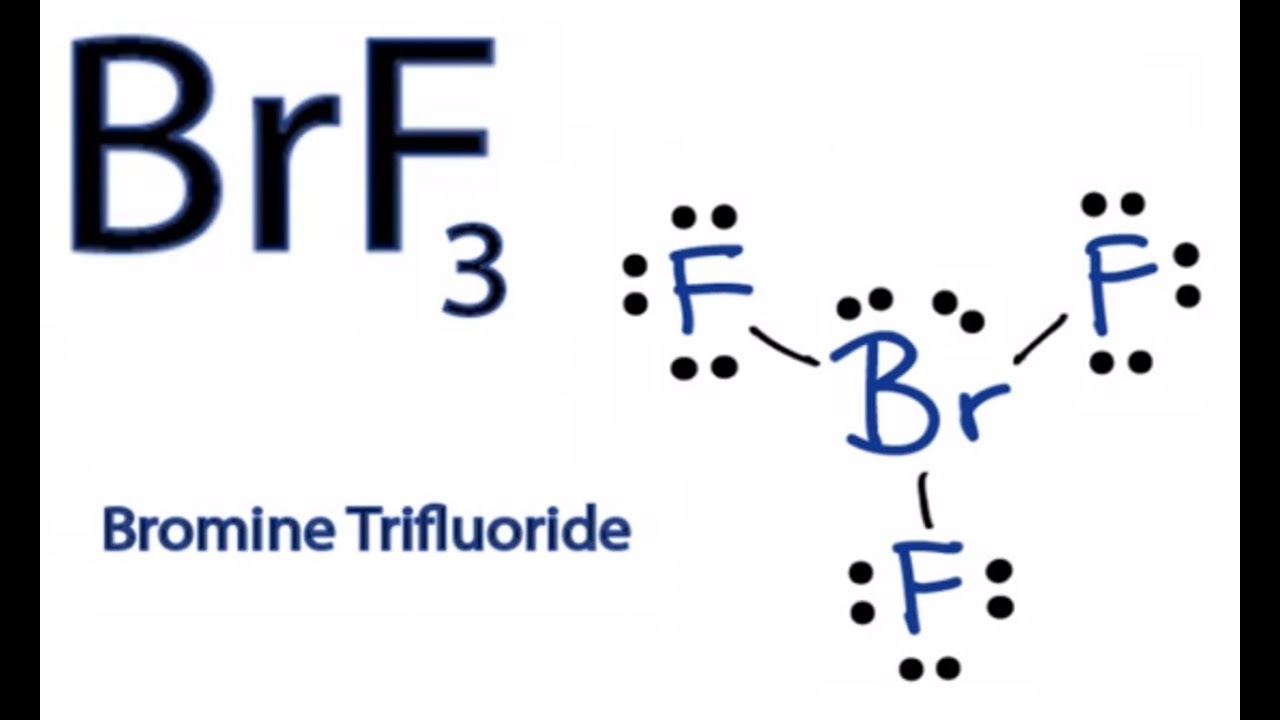
Electronegativity Of Bromine Trifluoride . Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. You can also use our tool as an. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. But it is due to the 2 lone pairs of. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. So, bromine should be placed in. It is represented as 1s2 2s22p6 3s23p63d104s24p5. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. After determining how many valence electrons there are in brf 3, place them. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. There are a total of 28 valence electrons for the brf 3 lewis structure.
from www.youtube.com
After determining how many valence electrons there are in brf 3, place them. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. It is represented as 1s2 2s22p6 3s23p63d104s24p5. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. But it is due to the 2 lone pairs of. So, bromine should be placed in. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. You can also use our tool as an.
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube
Electronegativity Of Bromine Trifluoride So, bromine should be placed in. It is represented as 1s2 2s22p6 3s23p63d104s24p5. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. After determining how many valence electrons there are in brf 3, place them. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. There are a total of 28 valence electrons for the brf 3 lewis structure. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. You can also use our tool as an. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. But it is due to the 2 lone pairs of. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. So, bromine should be placed in. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs.
From valenceelectrons.com
How to Find the Valence Electrons for BrF5? Electronegativity Of Bromine Trifluoride The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. But it is due to the 2 lone pairs of. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration.. Electronegativity Of Bromine Trifluoride.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Of Bromine Trifluoride The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. You can also use our tool as an. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs. Electronegativity Of Bromine Trifluoride.
From mavink.com
Periodic Table With Electronegativity Values Electronegativity Of Bromine Trifluoride Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. So, bromine should be placed in. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. Brf 3. Electronegativity Of Bromine Trifluoride.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Electronegativity Of Bromine Trifluoride There are a total of 28 valence electrons for the brf 3 lewis structure. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. Here in the brf3. Electronegativity Of Bromine Trifluoride.
From iperiodictable.com
What is Electronegativity Chart List of Electronegativity [PDF Electronegativity Of Bromine Trifluoride So, bromine should be placed in. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. There are a total of 28 valence electrons for the brf 3 lewis structure. Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs. Bromine flouride has. Electronegativity Of Bromine Trifluoride.
From ar.inspiredpencil.com
Bromine Trifluoride Electronegativity Of Bromine Trifluoride In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. But it is due to the 2 lone pairs of. It is represented. Electronegativity Of Bromine Trifluoride.
From cerwambr.blob.core.windows.net
Bromine Trifluoride Structure at Richard Moss blog Electronegativity Of Bromine Trifluoride After determining how many valence electrons there are in brf 3, place them. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. The ideal electron. Electronegativity Of Bromine Trifluoride.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Electronegativity Of Bromine Trifluoride You can also use our tool as an. After determining how many valence electrons there are in brf 3, place them. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. But it is due to the 2 lone pairs of. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. Each fluorine atom. Electronegativity Of Bromine Trifluoride.
From www.youtube.com
Is BrF3 (Bromine trifluoride) Ionic or Covalent/Molecular? YouTube Electronegativity Of Bromine Trifluoride Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. You can also use our tool as an. After determining how many valence electrons there are in brf 3, place them. The electronegativity calculator allows you to calculate the type of bond formed between different elements. Electronegativity Of Bromine Trifluoride.
From chemdictionary.org
Electronegativity Definition And Examples Chemistry Dictionary Electronegativity Of Bromine Trifluoride It is represented as 1s2 2s22p6 3s23p63d104s24p5. But it is due to the 2 lone pairs of. There are a total of 28 valence electrons for the brf 3 lewis structure. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. Bromine flouride has 28 valence electrons, which. Electronegativity Of Bromine Trifluoride.
From unacademy.com
What is the Hybridization of Bromine Pentafluoride Electronegativity Of Bromine Trifluoride The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. After determining how many valence electrons there. Electronegativity Of Bromine Trifluoride.
From www.ck12.org
Periodic Trends in Electronegativity CK12 Foundation Electronegativity Of Bromine Trifluoride It is represented as 1s2 2s22p6 3s23p63d104s24p5. But it is due to the 2 lone pairs of. So, bromine should be placed in. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. There are a total. Electronegativity Of Bromine Trifluoride.
From www.youtube.com
Hybridization of BrF3 (Bromine Trifluoride) YouTube Electronegativity Of Bromine Trifluoride It is represented as 1s2 2s22p6 3s23p63d104s24p5. But it is due to the 2 lone pairs of. You can also use our tool as an. So, bromine should be placed in. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. The ideal electron geometry of. Electronegativity Of Bromine Trifluoride.
From www.youtube.com
Lewis Structure of BrF3 (bromine trifluoride) YouTube Electronegativity Of Bromine Trifluoride Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. There are a total of 28 valence electrons for the brf 3 lewis structure.. Electronegativity Of Bromine Trifluoride.
From www.chemtube3d.com
BrF3 Bromine trifluoride Electronegativity Of Bromine Trifluoride The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. In the brf 3 lewis structure, there are three single bonds around the. Electronegativity Of Bromine Trifluoride.
From sciedutut.com
BrF5 Molecular Geometry Science Education and Tutorials Electronegativity Of Bromine Trifluoride It is represented as 1s2 2s22p6 3s23p63d104s24p5. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. After determining how many valence electrons there are in brf 3, place them. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. Each fluorine atom has three lone. Electronegativity Of Bromine Trifluoride.
From www.thoughtco.com
Printable Periodic Table of the Elements Electronegativity Electronegativity Of Bromine Trifluoride But it is due to the 2 lone pairs of. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. There are a total of 28. Electronegativity Of Bromine Trifluoride.
From in.pinterest.com
Bromine trifluoride has the chemical formula BrF3 and interhalogen Electronegativity Of Bromine Trifluoride There are a total of 28 valence electrons for the brf 3 lewis structure. After determining how many valence electrons there are in brf 3, place them. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. The hybridization of the central atom is sp3d, but. Electronegativity Of Bromine Trifluoride.
From www.alamy.com
Bromine chemical element with first ionization energy, atomic mass and Electronegativity Of Bromine Trifluoride But it is due to the 2 lone pairs of. It is represented as 1s2 2s22p6 3s23p63d104s24p5. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. So, bromine should be placed in. Each. Electronegativity Of Bromine Trifluoride.
From chem.libretexts.org
10.5 Bromine Trifluoride as a Solvent Chemistry LibreTexts Electronegativity Of Bromine Trifluoride After determining how many valence electrons there are in brf 3, place them. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. It is represented as 1s2 2s22p6 3s23p63d104s24p5. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. The. Electronegativity Of Bromine Trifluoride.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Electronegativity Of Bromine Trifluoride So, bromine should be placed in. After determining how many valence electrons there are in brf 3, place them. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal. Electronegativity Of Bromine Trifluoride.
From www.ch.imperial.ac.uk
VSEPR Theory A closer look at bromine trifluoride, BrF3. Henry Rzepa Electronegativity Of Bromine Trifluoride You can also use our tool as an. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. So, bromine should be placed. Electronegativity Of Bromine Trifluoride.
From quizlet.com
What is the hybridization of the central atom in the bromine Quizlet Electronegativity Of Bromine Trifluoride There are a total of 28 valence electrons for the brf 3 lewis structure. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. It is represented as 1s2 2s22p6 3s23p63d104s24p5. In the brf 3 lewis structure, there are three single bonds. Electronegativity Of Bromine Trifluoride.
From www.youtube.com
BrF3 Bromine Trifluoride Shape Hybridisation VSEPR Problem Electronegativity Of Bromine Trifluoride The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. But it is due to the 2 lone pairs of. After determining how many valence electrons there are in brf 3, place them. The electronegativity calculator allows you to calculate the type. Electronegativity Of Bromine Trifluoride.
From mungfali.com
Electronegativity Trend Periodic Table Electronegativity Of Bromine Trifluoride The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Bromine flouride has 28 valence electrons, which result in forming three bonds in the molecule and two lone pairs of electrons on the bromine atom. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone. Electronegativity Of Bromine Trifluoride.
From wellcometreeoflife.org
BrF3 Molecular Geometry (2021) Everything You Need to Know Electronegativity Of Bromine Trifluoride The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. There are a total of 28 valence electrons for the brf 3 lewis structure. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal. Electronegativity Of Bromine Trifluoride.
From www.numerade.com
SOLVED What volume of bromine is produced when 94.4 liters of bromine Electronegativity Of Bromine Trifluoride After determining how many valence electrons there are in brf 3, place them. It is represented as 1s2 2s22p6 3s23p63d104s24p5. You can also use our tool as an. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the. Electronegativity Of Bromine Trifluoride.
From whatsinsight.org
BrF3 (Bromine trifluoride) Molecular Geometry, Bond Angles What's Insight Electronegativity Of Bromine Trifluoride Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs. It is represented as 1s2 2s22p6 3s23p63d104s24p5. But it is due to the 2 lone pairs of. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it. The hybridization of the central atom. Electronegativity Of Bromine Trifluoride.
From www.chegg.com
Solved Name bromine trifluoride ∇ Electronegativity Of Bromine Trifluoride The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. It is represented as 1s2 2s22p6 3s23p63d104s24p5. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. There are a total of 28 valence electrons for the brf 3 lewis structure. But. Electronegativity Of Bromine Trifluoride.
From techiescientist.com
BrF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Electronegativity Of Bromine Trifluoride The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. After determining how many valence electrons there are in brf 3, place them. It is represented as 1s2 2s22p6 3s23p63d104s24p5. The ideal electron geometry of the bromine trifluoride (brf 3) molecule is. Electronegativity Of Bromine Trifluoride.
From cadscaleschart.z28.web.core.windows.net
electronegativity chart scale The periodic table and periodic trends Electronegativity Of Bromine Trifluoride After determining how many valence electrons there are in brf 3, place them. To determine the hybridization of bromine trifluoride, let’s first take the bromine atom, the central atom, and look at its electron configuration. It is represented as 1s2 2s22p6 3s23p63d104s24p5. So, bromine should be placed in. The hybridization of the central atom is sp3d, but to minimize the. Electronegativity Of Bromine Trifluoride.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube Electronegativity Of Bromine Trifluoride The ideal electron geometry of the bromine trifluoride (brf 3) molecule is trigonal bipyramidal. Here in the brf3 molecule, if we compare the bromine atom (br) and fluorine atom (f), then the bromine is less electronegative than fluorine. In the brf 3 lewis structure, there are three single bonds around the bromine atom, with three fluorine atoms attached to it.. Electronegativity Of Bromine Trifluoride.
From mavink.com
Printable Electronegativity Table Electronegativity Of Bromine Trifluoride So, bromine should be placed in. Each fluorine atom has three lone pairs, and the bromine atom has two lone pairs. The hybridization of the central atom is sp3d, but to minimize the repulsion between the lone pairs, the shape of the molecule is bent instead of trigonal pyramidal. Bromine flouride has 28 valence electrons, which result in forming three. Electronegativity Of Bromine Trifluoride.
From techiescientist.com
BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Electronegativity Of Bromine Trifluoride You can also use our tool as an. After determining how many valence electrons there are in brf 3, place them. But it is due to the 2 lone pairs of. So, bromine should be placed in. There are a total of 28 valence electrons for the brf 3 lewis structure. Brf 3 (bromine trifluoride) has one bromine atom and. Electronegativity Of Bromine Trifluoride.
From studyafrikander.z13.web.core.windows.net
How To Determine Electronegativity Electronegativity Of Bromine Trifluoride It is represented as 1s2 2s22p6 3s23p63d104s24p5. Brf 3 (bromine trifluoride) has one bromine atom and three fluorine atoms. There are a total of 28 valence electrons for the brf 3 lewis structure. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. In the brf 3 lewis structure, there are. Electronegativity Of Bromine Trifluoride.