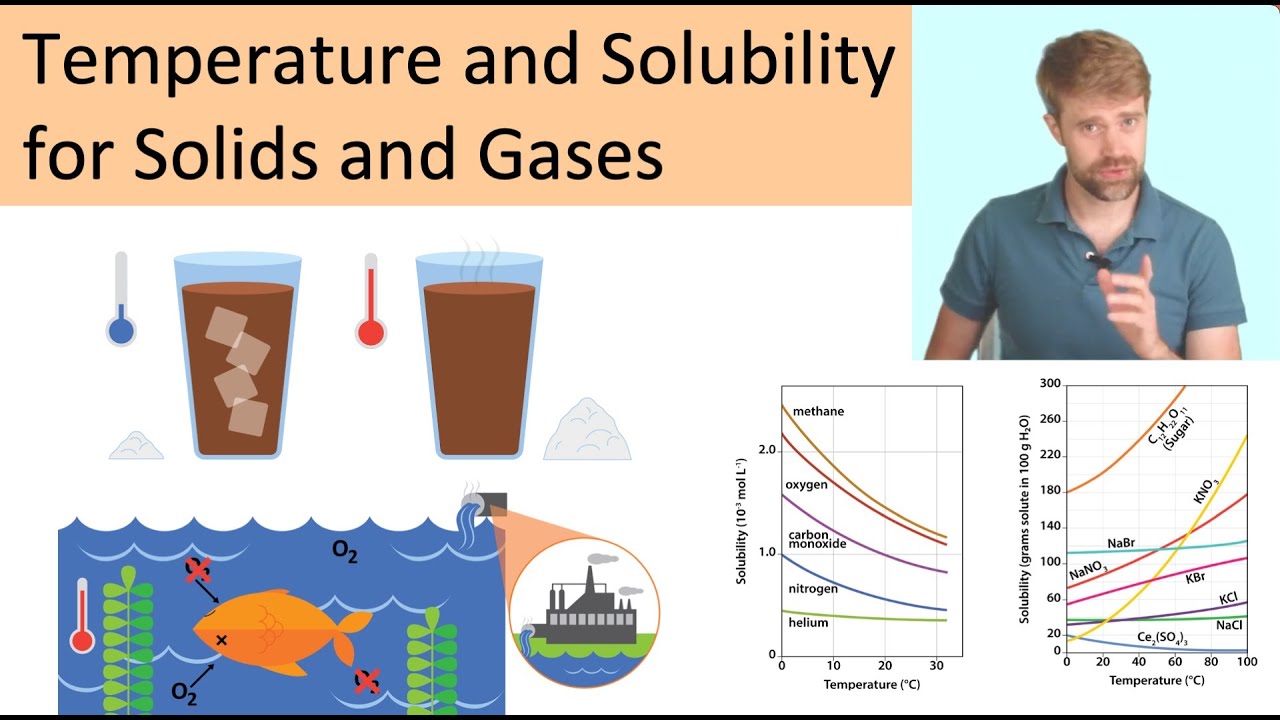
What Is Relationship Between Gas Solubility And Temperature . To understand the relationship among temperature, pressure, and solubility. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. Conversely, adding heat to the solution provides. This is due to the increased kinetic energy enabling the gas. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. Several factors affect the solubility of gases: We can use the second law of thermodynamics to. In general, solubility of a gas in water will decrease with. The solubility of gases in liquids decreases with increasing temperature. One of these factors is temperature. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. The solubility of a gas decreases with increasing temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas.
from www.youtube.com
The solubility of gases in liquids decreases with increasing temperature. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. Henry’s law describes the relationship between the pressure and the solubility of a gas. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. To understand the relationship among temperature, pressure, and solubility. One of these factors is temperature. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. In general, solubility of a gas in water will decrease with. Conversely, adding heat to the solution provides. Several factors affect the solubility of gases:
Temperature and Solubility Solids and Gases YouTube
What Is Relationship Between Gas Solubility And Temperature As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. Conversely, adding heat to the solution provides. We can use the second law of thermodynamics to. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. The solubility of gases in liquids decreases with increasing temperature. In general, solubility of a gas in water will decrease with. The solubility of a gas decreases with increasing temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. Several factors affect the solubility of gases: Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. This is due to the increased kinetic energy enabling the gas. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. One of these factors is temperature. To understand the relationship among temperature, pressure, and solubility. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID5798630 What Is Relationship Between Gas Solubility And Temperature This is due to the increased kinetic energy enabling the gas. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. In general, solubility of a gas in water will decrease with. We can use the second law of thermodynamics to. Gas solubility, a key concept in chemistry, refers to the ability of a. What Is Relationship Between Gas Solubility And Temperature.
From www.doubtnut.com
Solubility of oxygen gas in water follows Henry's law. When the solubi What Is Relationship Between Gas Solubility And Temperature Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. Several factors affect the solubility of gases: The solubility of gases in liquids decreases with increasing temperature. In general, solubility of a gas in water will decrease with. To understand the relationship among temperature, pressure, and solubility. As the kinetic energy of the gaseous. What Is Relationship Between Gas Solubility And Temperature.
From saylordotorg.github.io
Effects of Temperature and Pressure on Solubility What Is Relationship Between Gas Solubility And Temperature Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. Several factors affect the solubility of gases: The solubility of gases in liquids decreases with increasing temperature. Conversely, adding. What Is Relationship Between Gas Solubility And Temperature.
From classnotes.org.in
Solubility of Gases and Solids in Liquids Chemistry, Class 12, Solutions What Is Relationship Between Gas Solubility And Temperature One of these factors is temperature. The solubility of a gas decreases with increasing temperature. This is due to the increased kinetic energy enabling the gas. Henry’s law describes the relationship between the pressure and the solubility of a gas. To understand the relationship among temperature, pressure, and solubility. Gas solubility, a key concept in chemistry, refers to the ability. What Is Relationship Between Gas Solubility And Temperature.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 What Is Relationship Between Gas Solubility And Temperature Conversely, adding heat to the solution provides. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. One of these factors is temperature. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. To understand the relationship among. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID5581895 What Is Relationship Between Gas Solubility And Temperature The solubility of a gas decreases with increasing temperature. In general, solubility of a gas in water will decrease with. This is due to the increased kinetic energy enabling the gas. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. The solubility. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Is Relationship Between Gas Solubility And Temperature To understand the relationship among temperature, pressure, and solubility. One of these factors is temperature. We can use the second law of thermodynamics to. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. The solubility of a gas decreases with increasing temperature. Several factors affect the solubility of gases: Conversely, adding heat to the. What Is Relationship Between Gas Solubility And Temperature.
From tinnongtuyensinh.com
Exploring Temperatures Impact On Solubility Solid Vs. Gas What Is Relationship Between Gas Solubility And Temperature Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free What Is Relationship Between Gas Solubility And Temperature As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. Henry’s law describes the relationship between the pressure and the solubility of a gas. Conversely, adding heat to the solution provides. One of these factors is temperature. The solubility of a gas decreases with increasing temperature. This is due to the increased kinetic energy enabling. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation What Is Relationship Between Gas Solubility And Temperature We can use the second law of thermodynamics to. The solubility of gases in liquids decreases with increasing temperature. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. The understand that the solubility of a solid may increase or decrease with increasing temperature, to.. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility vs. Temperature PowerPoint Presentation, free download What Is Relationship Between Gas Solubility And Temperature Several factors affect the solubility of gases: Conversely, adding heat to the solution provides. The solubility of a gas decreases with increasing temperature. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. This is due to the increased kinetic energy enabling the gas. The understand that the solubility of a solid may increase or. What Is Relationship Between Gas Solubility And Temperature.
From www.youtube.com
Henry’s law. Solubility of gas solute. Effect of temperature and What Is Relationship Between Gas Solubility And Temperature Conversely, adding heat to the solution provides. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. In general, solubility of a gas in water will decrease with. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free What Is Relationship Between Gas Solubility And Temperature As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. The solubility of a gas decreases with increasing temperature. Conversely, adding heat to the solution provides. Henry’s law describes the relationship between the pressure and the solubility of a gas. In general, solubility of a gas in water will decrease with. Several factors affect the. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Chapter 9 Solutions PowerPoint Presentation, free download ID What Is Relationship Between Gas Solubility And Temperature The solubility of gases in liquids decreases with increasing temperature. In general, solubility of a gas in water will decrease with. The solubility of a gas decreases with increasing temperature. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. We can use. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID1987861 What Is Relationship Between Gas Solubility And Temperature Henry’s law describes the relationship between the pressure and the solubility of a gas. The solubility of gases in liquids decreases with increasing temperature. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. The solubility of a gas decreases with increasing temperature. To understand the relationship among temperature, pressure, and solubility. The understand. What Is Relationship Between Gas Solubility And Temperature.
From www.youtube.com
6 Gas Solubility YouTube What Is Relationship Between Gas Solubility And Temperature This is due to the increased kinetic energy enabling the gas. The solubility of a gas decreases with increasing temperature. In general, solubility of a gas in water will decrease with. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. Several factors. What Is Relationship Between Gas Solubility And Temperature.
From drubarrett.blogspot.com
What Relationship Exists Between Solubility And Temperature For Most Of What Is Relationship Between Gas Solubility And Temperature To understand the relationship among temperature, pressure, and solubility. One of these factors is temperature. In general, solubility of a gas in water will decrease with. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid. What Is Relationship Between Gas Solubility And Temperature.
From mungfali.com
Gas Solubility Curve What Is Relationship Between Gas Solubility And Temperature The understand that the solubility of a solid may increase or decrease with increasing temperature, to. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. In general, solubility of a gas in water will decrease with. One of these factors is temperature. Contrary to. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 What Is Relationship Between Gas Solubility And Temperature Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. Conversely, adding heat to the solution provides. Henry’s law describes the relationship between the pressure and the solubility of. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID6014910 What Is Relationship Between Gas Solubility And Temperature As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. The solubility of gases in liquids decreases with increasing temperature. One of these factors is temperature. This is due to the increased kinetic energy enabling the gas. The understand that the solubility of. What Is Relationship Between Gas Solubility And Temperature.
From www.ck12.org
Gas Solubility (use graphs) Overview ( Video ) Chemistry CK12 What Is Relationship Between Gas Solubility And Temperature The solubility of a gas decreases with increasing temperature. Conversely, adding heat to the solution provides. The solubility of gases in liquids decreases with increasing temperature. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. Contrary to solids, the solubility of gases. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free What Is Relationship Between Gas Solubility And Temperature One of these factors is temperature. Several factors affect the solubility of gases: To understand the relationship among temperature, pressure, and solubility. Conversely, adding heat to the solution provides. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas. The. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download What Is Relationship Between Gas Solubility And Temperature Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. The solubility of gases in liquids decreases with increasing temperature. The solubility of a gas decreases with increasing temperature. To understand the relationship among temperature, pressure, and solubility. This. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility vs. Temperature PowerPoint Presentation, free download What Is Relationship Between Gas Solubility And Temperature We can use the second law of thermodynamics to. Several factors affect the solubility of gases: In general, solubility of a gas in water will decrease with. The solubility of a gas decreases with increasing temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas. The solubility of gases in liquids decreases with increasing temperature.. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID6751365 What Is Relationship Between Gas Solubility And Temperature This is due to the increased kinetic energy enabling the gas. One of these factors is temperature. Several factors affect the solubility of gases: Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. As the kinetic energy of the gaseous solute increases, its molecules. What Is Relationship Between Gas Solubility And Temperature.
From www.researchgate.net
CO2 solubility as a function of water depth (m) and temperature (°C What Is Relationship Between Gas Solubility And Temperature In general, solubility of a gas in water will decrease with. The solubility of gases in liquids decreases with increasing temperature. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. Henry’s law describes the relationship between the pressure. What Is Relationship Between Gas Solubility And Temperature.
From www.youtube.com
The Effect of Temperature and Pressure on Solubility Mr C YouTube What Is Relationship Between Gas Solubility And Temperature Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. As the temperature of a liquid increases, the solubilities of gases in that liquid decrease. In general, solubility of a gas in water will decrease with. The solubility of a gas decreases with increasing temperature.. What Is Relationship Between Gas Solubility And Temperature.
From www.youtube.com
Temperature and Solubility Solids and Gases YouTube What Is Relationship Between Gas Solubility And Temperature To understand the relationship among temperature, pressure, and solubility. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. We can use the second law of thermodynamics to. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. Conversely,. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 What Is Relationship Between Gas Solubility And Temperature The solubility of a gas decreases with increasing temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. The solubility of gases in liquids decreases with increasing temperature. Conversely, adding heat to the solution provides. We can use the. What Is Relationship Between Gas Solubility And Temperature.
From www.researchgate.net
Gassolubility pressure diagram. Download Scientific Diagram What Is Relationship Between Gas Solubility And Temperature The solubility of a gas decreases with increasing temperature. In general, solubility of a gas in water will decrease with. This is due to the increased kinetic energy enabling the gas. We can use the second law of thermodynamics to. The understand that the solubility of a solid may increase or decrease with increasing temperature, to. The solubility of gases. What Is Relationship Between Gas Solubility And Temperature.
From courses.lumenlearning.com
Gas Solubility and Temperature Introduction to Chemistry What Is Relationship Between Gas Solubility And Temperature The solubility of gases in liquids decreases with increasing temperature. Henry’s law describes the relationship between the pressure and the solubility of a gas. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. One of these factors is temperature. Conversely, adding heat to the solution provides. We can use the second law of. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Chapter 7 Solutions and Colloids PowerPoint Presentation, free What Is Relationship Between Gas Solubility And Temperature Several factors affect the solubility of gases: In general, solubility of a gas in water will decrease with. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. We can use the second law of thermodynamics to. The understand that the solubility of a solid. What Is Relationship Between Gas Solubility And Temperature.
From www.slideserve.com
PPT Chapter 11 Measuring Solubility PowerPoint Presentation, free What Is Relationship Between Gas Solubility And Temperature One of these factors is temperature. Contrary to solids, the solubility of gases in liquids typically decreases with an increase in temperature. To understand the relationship among temperature, pressure, and solubility. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific conditions of temperature and. The solubility of a. What Is Relationship Between Gas Solubility And Temperature.
From slideplayer.com
Reading Solubility Curves ppt download What Is Relationship Between Gas Solubility And Temperature The understand that the solubility of a solid may increase or decrease with increasing temperature, to. Henry’s law describes the relationship between the pressure and the solubility of a gas. We can use the second law of thermodynamics to. Gas solubility, a key concept in chemistry, refers to the ability of a gas to dissolve in a liquid under specific. What Is Relationship Between Gas Solubility And Temperature.
From www.chegg.com
Solved Use the graph below to describe the general What Is Relationship Between Gas Solubility And Temperature The understand that the solubility of a solid may increase or decrease with increasing temperature, to. As the kinetic energy of the gaseous solute increases, its molecules have a greater tendency to escape the attraction of the solvent molecules and return to the. This is due to the increased kinetic energy enabling the gas. Gas solubility, a key concept in. What Is Relationship Between Gas Solubility And Temperature.