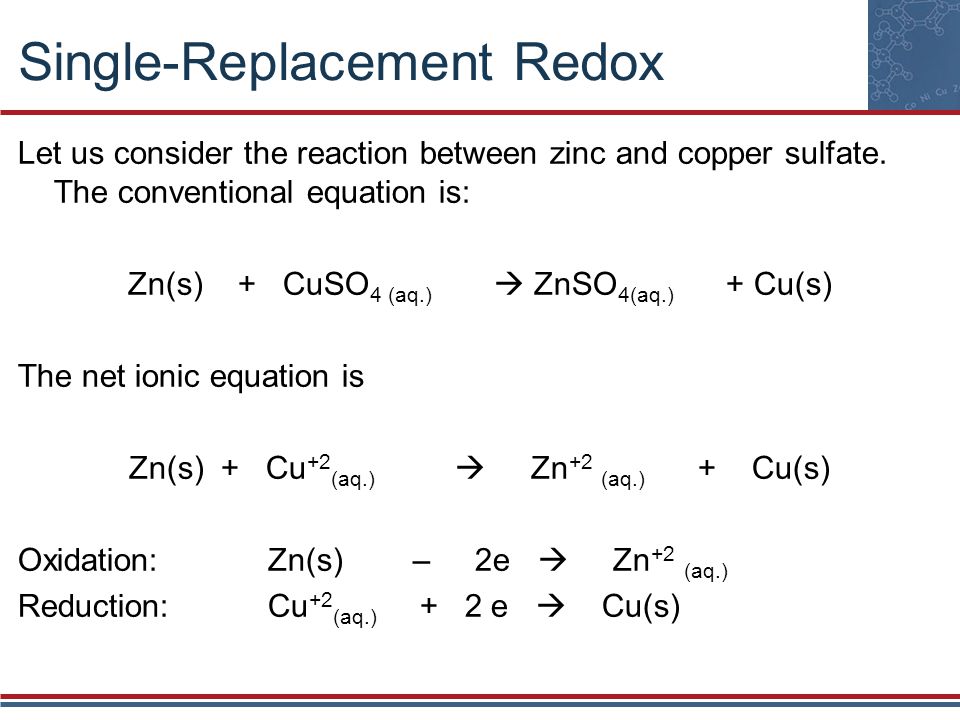
Zinc Sulfate Equation . This is because magnesium could displace copper and zinc, zinc could only. What is the formula of the hydrated zinc sulphate? A labelled diagram is acceptable. The order of reactivity is: Water of crystallisation describes water molecules that are bonded into a crystalline. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. It is on the world health organization's list of essential medicines, a. Magnesium > zinc > copper. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction.
from schoolworkhelper.net
Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Magnesium > zinc > copper. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. A labelled diagram is acceptable. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen What is the formula of the hydrated zinc sulphate? Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. The order of reactivity is: It is on the world health organization's list of essential medicines, a.
Single Displacement Reactions Lab Explained SchoolWorkHelper
Zinc Sulfate Equation Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. It is on the world health organization's list of essential medicines, a. What is the formula of the hydrated zinc sulphate? The order of reactivity is: Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Water of crystallisation describes water molecules that are bonded into a crystalline. This is because magnesium could displace copper and zinc, zinc could only. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. A labelled diagram is acceptable. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.
From www.slideshare.net
Replacement reactions Zinc Sulfate Equation A labelled diagram is acceptable. What is the formula of the hydrated zinc sulphate? Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Water of crystallisation describes water molecules that are bonded. Zinc Sulfate Equation.
From www.youtube.com
How to Find the Percent Composition of Zn in ZnSO4 (Zinc Sulfate) YouTube Zinc Sulfate Equation Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Magnesium > zinc > copper. Water of crystallisation describes water molecules that are bonded into a crystalline. This is because magnesium could displace copper and zinc, zinc could only. Acids will react with reactive metals, such as magnesium and zinc, to make a. Zinc Sulfate Equation.
From www.youtube.com
how to Write Zinc Sulphate formula Molecular formula of Zinc Sulphate Zinc Sulfate Equation A labelled diagram is acceptable. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Magnesium > zinc > copper. What is the formula of the hydrated zinc sulphate? Water of crystallisation describes water molecules that are bonded into a crystalline. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis.. Zinc Sulfate Equation.
From molekula.com
Purchase Zinc sulfate heptahydrate [7446200] online • Catalog Zinc Sulfate Equation Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. It is on the world health organization's list of essential medicines, a. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as. Zinc Sulfate Equation.
From www.alamy.com
Zinc sulfate is a molecular chemical formula. Zinc infographics. Vector Zinc Sulfate Equation Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Magnesium > zinc > copper. Water of crystallisation describes water molecules that are bonded into a crystalline. This is because magnesium could displace copper and zinc, zinc. Zinc Sulfate Equation.
From www.youtube.com
H2SO4+Zn=ZnSO4+H2 Balanced EquationSulphuric acid+Zinc=Zinc sulphate Zinc Sulfate Equation Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. It is on the world health organization's list of essential medicines, a. Use half equations to explain why the reaction of zinc with sulfuric acid is. Zinc Sulfate Equation.
From www.glentham.com
Zinc sulfate monohydrate (CAS 7446197) Glentham Life Sciences Zinc Sulfate Equation Water of crystallisation describes water molecules that are bonded into a crystalline. What is the formula of the hydrated zinc sulphate? Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. The order of reactivity is: A. Zinc Sulfate Equation.
From beautyambassade.com
Zinc Sulfate Skincare ingredient Skin care products Zinc Sulfate Equation Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Magnesium > zinc > copper. This is because magnesium could displace copper and zinc, zinc could only. It is on the world health organization's list of essential medicines, a. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Acids. Zinc Sulfate Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc Sulfate Equation Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. This is because magnesium could displace copper and zinc, zinc could only. Water of crystallisation describes water molecules that are bonded into a crystalline. Magnesium > zinc > copper. What is the formula of the hydrated zinc sulphate? Acid + metal → salt + hydrogen hydrochloric acid. Zinc Sulfate Equation.
From www.fishersci.se
Zinc Sulfate Solution, 0.1M, Honeywell Fisher Scientific Zinc Sulfate Equation What is the formula of the hydrated zinc sulphate? Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium > zinc > copper. It is on the world health organization's list of essential medicines, a. Water of crystallisation describes water molecules that are bonded into a crystalline. Describe how zinc metal can. Zinc Sulfate Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Sulfate Equation Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. The order of reactivity is: Acids will react with reactive metals, such as magnesium. Zinc Sulfate Equation.
From www.shutterstock.com
Zinc Sulfate Compound Formula Znso4 库存插图 769733398 Shutterstock Zinc Sulfate Equation Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Magnesium > zinc > copper. The order of reactivity is: Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen It is on the world health organization's list of essential medicines, a. This is because magnesium could. Zinc Sulfate Equation.
From www.dreamstime.com
3D Image of Zinc Sulfate Skeletal Formula Stock Illustration Zinc Sulfate Equation Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. What is the formula of the hydrated zinc sulphate? It is on the world health organization's list of essential medicines, a. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This. Zinc Sulfate Equation.
From www.alamy.com
Zinc sulfate is a molecular chemical formula. Zinc infographics. Vector Zinc Sulfate Equation Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Water of crystallisation describes water molecules that are bonded into. Zinc Sulfate Equation.
From cartoondealer.com
Zinc Sulfate Is A Molecular Chemical Formula. Zinc Infographics. Vector Zinc Sulfate Equation Magnesium > zinc > copper. Water of crystallisation describes water molecules that are bonded into a crystalline. The order of reactivity is: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. A labelled diagram is acceptable. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. It is on. Zinc Sulfate Equation.
From ceotvsix.blob.core.windows.net
Magnesium And Zinc Sulfate Word Equation at Scott Winbush blog Zinc Sulfate Equation Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. A labelled diagram is acceptable. Water of crystallisation describes water molecules that are bonded into. Zinc Sulfate Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Sulfate Equation The order of reactivity is: This is because magnesium could displace copper and zinc, zinc could only. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Water of crystallisation describes water molecules that are bonded into a crystalline. Magnesium > zinc > copper. Describe how zinc metal can be obtained from zinc. Zinc Sulfate Equation.
From www.t3db.ca
T3DB Zinc sulfate Zinc Sulfate Equation Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Water of crystallisation describes water molecules that are bonded into a crystalline. What is the formula of the hydrated zinc sulphate? Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Describe how zinc metal can be. Zinc Sulfate Equation.
From brainly.com
15.00g of hydrated zinc sulfate loses 6.579 H2O during heating what is Zinc Sulfate Equation It is on the world health organization's list of essential medicines, a. Magnesium > zinc > copper. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. What is the formula of the hydrated zinc sulphate? This is because magnesium could displace copper and zinc, zinc could only. A labelled diagram is acceptable.. Zinc Sulfate Equation.
From www.alamy.com
Zinc sulfate, chemical structure. Skeletal formula Stock Vector Image Zinc Sulfate Equation Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. What is the formula of the hydrated zinc sulphate? The order of reactivity is: A labelled diagram is acceptable. This is because magnesium could displace copper and zinc, zinc could only. It is on the world health organization's list of essential medicines, a. Water. Zinc Sulfate Equation.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Sulfate Equation Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. The order of reactivity is: What is the formula of the hydrated zinc sulphate? Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Acids will react with reactive metals, such as magnesium and zinc, to. Zinc Sulfate Equation.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Zinc Sulfate Equation What is the formula of the hydrated zinc sulphate? Water of crystallisation describes water molecules that are bonded into a crystalline. This is because magnesium could displace copper and zinc, zinc could only. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Describe how zinc metal can be obtained from zinc sulfate. Zinc Sulfate Equation.
From brainly.in
write the chemical formula of the ZINC SULPHATE using Criss cross Zinc Sulfate Equation Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen A labelled diagram is acceptable. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. This is because magnesium could displace copper and zinc, zinc. Zinc Sulfate Equation.
From www.dreamstime.com
Zinc Sulfate is a Molecular Chemical Formula. Zinc Infographics. Vector Zinc Sulfate Equation It is on the world health organization's list of essential medicines, a. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. A. Zinc Sulfate Equation.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Zinc Sulfate Equation Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen A labelled diagram is acceptable. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. It is on the world health organization's list of essential. Zinc Sulfate Equation.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc Sulfate Equation What is the formula of the hydrated zinc sulphate? Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen This is because magnesium could displace copper and zinc, zinc could only. Water of crystallisation describes water molecules that are bonded into. Zinc Sulfate Equation.
From www.bio-world.com
Zinc Sulfate Heptahydrate (7446200) bioWORLD Zinc Sulfate Equation Water of crystallisation describes water molecules that are bonded into a crystalline. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This is. Zinc Sulfate Equation.
From brainly.in
zinc sulphate formulae by criss cross method Brainly.in Zinc Sulfate Equation A labelled diagram is acceptable. Magnesium > zinc > copper. Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Water of crystallisation describes water molecules that are bonded into a crystalline. What is the formula of. Zinc Sulfate Equation.
From www.shutterstock.com
Zinc Sulfate Chemical Structure Skeletal Formula Stock Vector (Royalty Zinc Sulfate Equation A labelled diagram is acceptable. Water of crystallisation describes water molecules that are bonded into a crystalline. It is on the world health organization's list of essential medicines, a. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. What is the formula of the hydrated zinc sulphate? Describe how zinc metal can. Zinc Sulfate Equation.
From cartoondealer.com
Zinc Sulfate, Chemical Structure. Skeletal Formula. Vector Illustration Zinc Sulfate Equation Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. This is because magnesium could displace copper and zinc, zinc could only. Water of crystallisation describes water molecules that are bonded into a crystalline. It is on the world health organization's list of essential medicines, a. Magnesium > zinc > copper. What is the. Zinc Sulfate Equation.
From www.numerade.com
SOLVEDBalance the equation for the reaction in which hot, concentrated Zinc Sulfate Equation It is on the world health organization's list of essential medicines, a. Magnesium > zinc > copper. The order of reactivity is: Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Use half equations to explain why the reaction. Zinc Sulfate Equation.
From www.dreamstime.com
Zinc Sulfate, Great Design for Any Purposes. Zinc Sulfate Formula Zinc Sulfate Equation Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. This is because magnesium could displace copper and zinc, zinc could only. Acids will. Zinc Sulfate Equation.
From www.shutterstock.com
Zinc Sulfate Formula Handwritten Chemical Formula Stock Illustration Zinc Sulfate Equation The order of reactivity is: Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. Use half equations to explain why the reaction of zinc with sulfuric acid is a redox reaction. Magnesium > zinc > copper. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.. Zinc Sulfate Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Sulfate Equation Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The order of reactivity is: What is the formula of the hydrated zinc sulphate? Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. Acid + metal → salt + hydrogen hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc Sulfate Equation.
From www.youtube.com
How to Balance H2SO4 + Zn = ZnSO4 + H2 (Sulfuric acid + Zinc) YouTube Zinc Sulfate Equation Zinc sulfate is the inorganic compound with the formula znso4 and historically known as white vitriol. This is because magnesium could displace copper and zinc, zinc could only. Describe how zinc metal can be obtained from zinc sulfate solution by electrolysis. What is the formula of the hydrated zinc sulphate? Acids will react with reactive metals, such as magnesium and. Zinc Sulfate Equation.