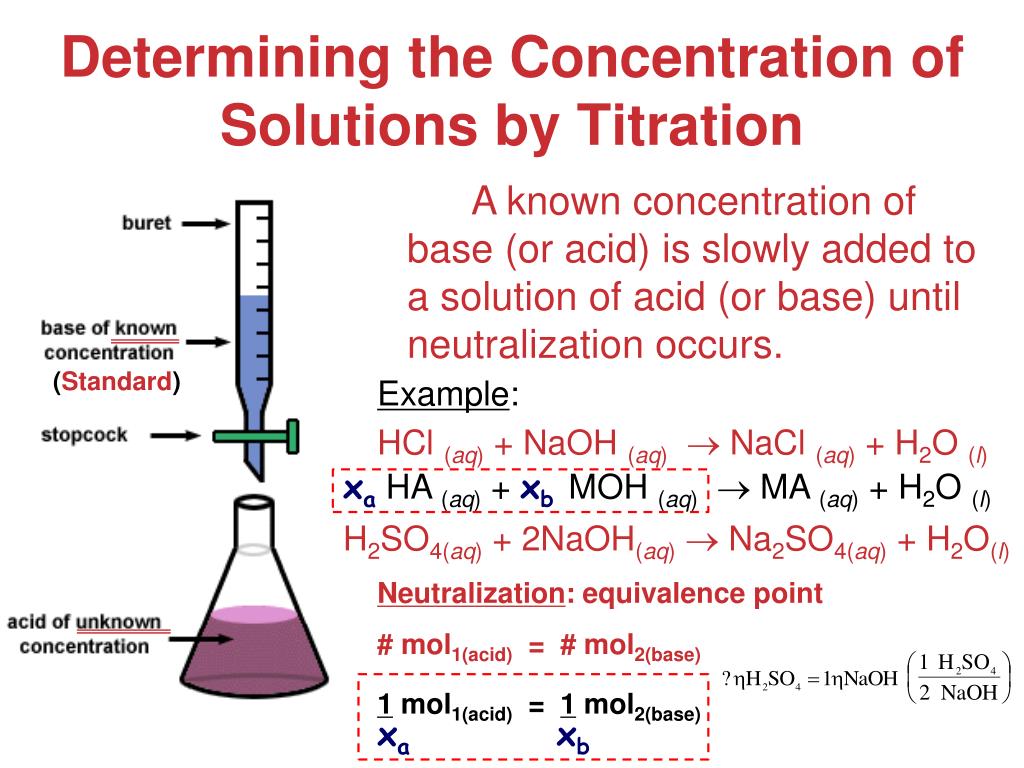
How To Solve Titration Problems In Chemistry . This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. It explains how to solve acid base titration problems using a formula and a conversion process in typical. What was the concentration of the hcl? Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. By the end of this section, you will be able to: Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain.
from www.slideserve.com
What was the concentration of the hcl? Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. It explains how to solve acid base titration problems using a formula and a conversion process in typical. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. By the end of this section, you will be able to:
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
How To Solve Titration Problems In Chemistry Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. What was the concentration of the hcl? Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. By the end of this section, you will be able to: Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. It explains how to solve acid base titration problems using a formula and a conversion process in typical. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution.
From www.youtube.com
Back Titration Example YouTube How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. This webpage describes a procedure called titration, which can be used to. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Molarity Chemistry Tutorial YouTube How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What was the concentration of the hcl? It explains how to solve acid base titration problems using a formula and a conversion process in typical. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a. How To Solve Titration Problems In Chemistry.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school How To Solve Titration Problems In Chemistry This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. It explains how to solve acid base titration problems using a formula and a conversion process in typical. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a. How To Solve Titration Problems In Chemistry.
From www.reddit.com
WB/SA Titration. Can someone please help me solve this problem. I’m not How To Solve Titration Problems In Chemistry What was the concentration of the hcl? The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. By the end of this section, you will be able to: Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Acidbase titration problem 1 YouTube How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. This webpage describes a procedure called titration, which can be used to find the. How To Solve Titration Problems In Chemistry.
From www.edplace.com
Understand Advanced Titration Calculations Worksheet EdPlace How To Solve Titration Problems In Chemistry It explains how to solve acid base titration problems using a formula and a conversion process in typical. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. What was the concentration of the hcl? Titrations are used to obtain quantitative. How To Solve Titration Problems In Chemistry.
From www.youtube.com
How to solve Ch11 HW problems on finding molarity TITRATION(GOB) YouTube How To Solve Titration Problems In Chemistry Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. It explains how to solve acid base titration problems using a formula and a conversion process in typical. The example below demonstrates the technique to solve a titration problem for a. How To Solve Titration Problems In Chemistry.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. It explains how to solve acid base titration problems using a formula and a conversion process in typical. Weak acid and strong base. How To Solve Titration Problems In Chemistry.
From noel-kmclean.blogspot.com
Calculate Molarity of Acid in Titration How To Solve Titration Problems In Chemistry Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. By the end of this section, you will be able to: This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. It. How To Solve Titration Problems In Chemistry.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE How To Solve Titration Problems In Chemistry This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. By the end of this section, you will be able to: What was the concentration of the hcl? It explains how to solve acid base titration problems using a formula and a conversion process in typical.. How To Solve Titration Problems In Chemistry.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog How To Solve Titration Problems In Chemistry Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. By the end of this section, you will be able to: This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. A. How To Solve Titration Problems In Chemistry.
From lessonlibepinicions.z22.web.core.windows.net
Chemistry Titration Questions And Answers How To Solve Titration Problems In Chemistry Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What was the concentration of the hcl? The example below demonstrates the technique to. How To Solve Titration Problems In Chemistry.
From www.slideserve.com
PPT Honors Chemistry Unit 7 Stoichiometry PowerPoint Presentation How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium. How To Solve Titration Problems In Chemistry.
From learningcampussplit.z21.web.core.windows.net
Titration Practice Problems With Answers Pdf How To Solve Titration Problems In Chemistry Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. What was the concentration of the hcl? Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. It explains how to solve acid base titration problems using a formula. How To Solve Titration Problems In Chemistry.
From mungfali.com
Titration Steps How To Solve Titration Problems In Chemistry Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. What was the concentration of the hcl? It explains how to solve acid base. How To Solve Titration Problems In Chemistry.
From www.casadeinonni.org
How To Solve A Redox Titration Problem Chemistry How To Solve Titration Problems In Chemistry Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. It explains how to solve acid base titration problems using a formula and a conversion process in typical. Titrations are used to obtain quantitative information about chemical reactions, such as the. How To Solve Titration Problems In Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Solve Titration Problems In Chemistry This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titrations are used to obtain quantitative information. How To Solve Titration Problems In Chemistry.
From tukioka-clinic.com
👍 Solve titration problems. How to solve titration problems step by How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. By the end of this section, you will be able to: Weak acid and strong base titration problems when. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Titrations HL How to solve IB chemistry problems in paper 1, part 35 How To Solve Titration Problems In Chemistry By the end of this section, you will be able to: A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. What was the concentration of the hcl? This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a. How To Solve Titration Problems In Chemistry.
From www.youtube.com
AP Chemistry Titration Graph problem worksheet review YouTube How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. It explains how to. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. By the end of this section, you will be able to: Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. The example below demonstrates the technique to solve a titration problem for a. How To Solve Titration Problems In Chemistry.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube How To Solve Titration Problems In Chemistry By the end of this section, you will be able to: A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Titrations are used to obtain quantitative information about chemical reactions, such as the. How To Solve Titration Problems In Chemistry.
From mavink.com
Acid Base Titration Problems How To Solve Titration Problems In Chemistry What was the concentration of the hcl? Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. It explains how to. How To Solve Titration Problems In Chemistry.
From www.wizeprep.com
Titration Problems Wize University Chemistry Textbook Wizeprep How To Solve Titration Problems In Chemistry It explains how to solve acid base titration problems using a formula and a conversion process in typical. What was the concentration of the hcl? This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. Use stoichiometry of the neutralization to determine the amounts of acid. How To Solve Titration Problems In Chemistry.
From ceavsgvd.blob.core.windows.net
How To Calculate Acid Concentration From Titration at Susan Carrier blog How To Solve Titration Problems In Chemistry Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid. How To Solve Titration Problems In Chemistry.
From exyfapawg.blob.core.windows.net
AcidBase Titration Multiple Choice Questions Pdf at Sharon Castillo blog How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. It explains how to solve acid base titration problems using a formula and a conversion process in. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Solving AcidBase Titration Problems YouTube How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. This webpage describes a procedure called titration, which can be used to. How To Solve Titration Problems In Chemistry.
From tukioka-clinic.com
👍 Solve titration problems. How to solve titration problems step by How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Titrations are used to obtain quantitative information about chemical reactions, such as the concentration. How To Solve Titration Problems In Chemistry.
From www.reddit.com
Titration Homework how do I solve this problem when no info about How To Solve Titration Problems In Chemistry This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. By the end of this section, you will be able to: Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that. How To Solve Titration Problems In Chemistry.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 How To Solve Titration Problems In Chemistry What was the concentration of the hcl? It explains how to solve acid base titration problems using a formula and a conversion process in typical. By the end of this section, you will be able to: This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base.. How To Solve Titration Problems In Chemistry.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. Weak acid and strong base titration problems when solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. The example below demonstrates the technique to solve a titration problem for. How To Solve Titration Problems In Chemistry.
From www.youtube.com
Titration sample problems finding volume YouTube How To Solve Titration Problems In Chemistry Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a. How To Solve Titration Problems In Chemistry.
From www.madebyteachers.com
Solution and Titration Stoichiometry a Chemistry Worksheet Made By How To Solve Titration Problems In Chemistry Titrations are used to obtain quantitative information about chemical reactions, such as the concentration of a solution or establish the stoichiometry of a reaction. It explains how to solve acid base titration problems using a formula and a conversion process in typical. This webpage describes a procedure called titration, which can be used to find the molarity of a solution. How To Solve Titration Problems In Chemistry.
From ar.inspiredpencil.com
Amphoteric Examples How To Solve Titration Problems In Chemistry This webpage describes a procedure called titration, which can be used to find the molarity of a solution of an acid or a base. It explains how to solve acid base titration problems using a formula and a conversion process in typical. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with. How To Solve Titration Problems In Chemistry.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Solve Titration Problems In Chemistry A 25 ml solution of 0.5 m naoh is titrated until neutralized into a 50 ml sample of hcl. The example below demonstrates the technique to solve a titration problem for a titration of sulfuric acid with sodium hydroxide. What was the concentration of the hcl? Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base. How To Solve Titration Problems In Chemistry.