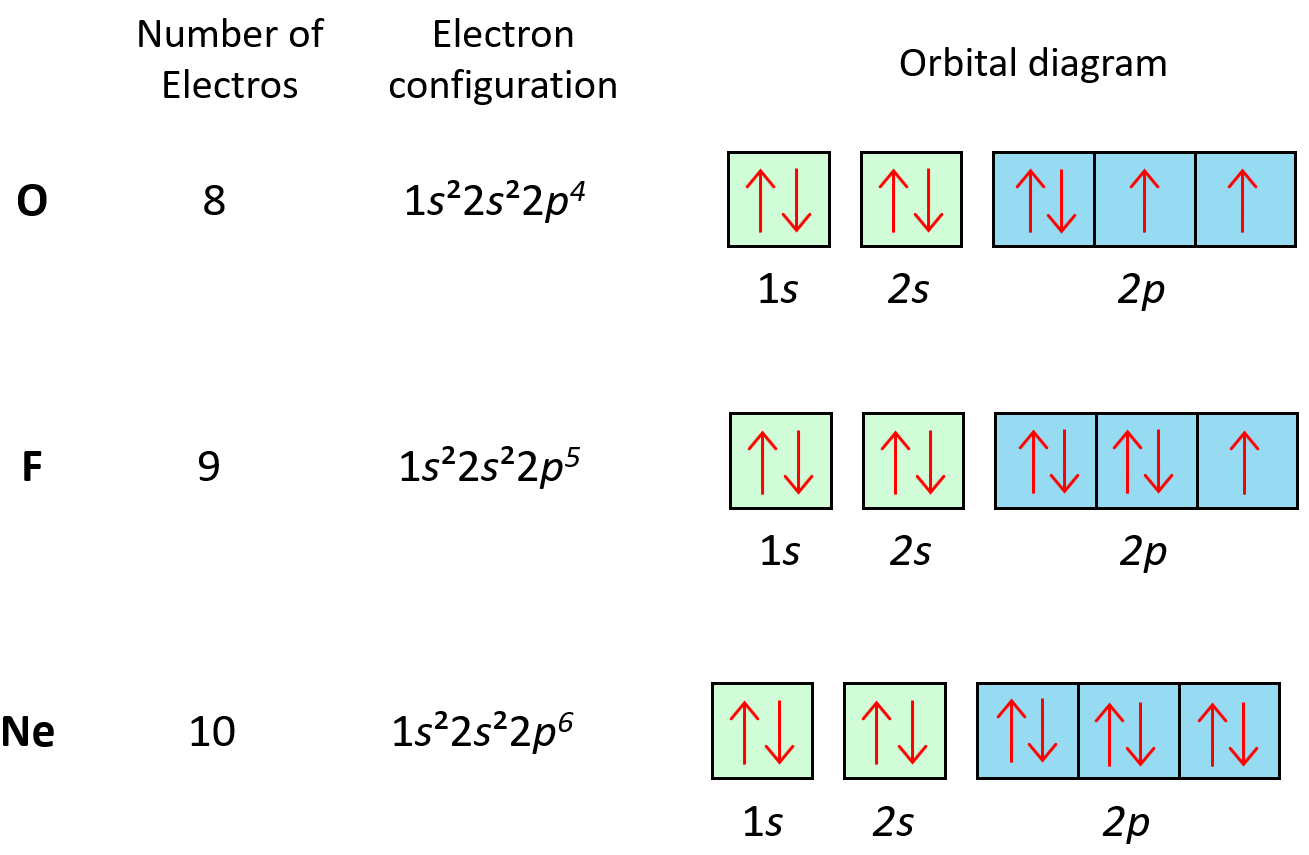
Nitric Oxide Electron Configuration . So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. One n atom, and one o atom. No, or nitric oxide has two atoms: Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. Therefore, total valence electrons in no= 5+6 = 11. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement.
from general.chemistrysteps.com
Therefore, total valence electrons in no= 5+6 = 11. The no+ cation, which is formed by. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. One n atom, and one o atom. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Here is the mo diagram for nitric oxide: No, or nitric oxide has two atoms: Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement.
Orbital Diagrams Chemistry Steps
Nitric Oxide Electron Configuration So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. No, or nitric oxide has two atoms: One n atom, and one o atom. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. Therefore, total valence electrons in no= 5+6 = 11.
From socratic.org
What is the electron configuration of nitrogen monoxide? Socratic Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. One n atom, and one o atom. Therefore, total valence electrons in no= 5+6 = 11. Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. So, the molecular electron configuration would be written in a similar manner. Nitric Oxide Electron Configuration.
From www.dreamstime.com
Nitric Oxide NO Free Radical and Signaling Molecule. Skeletal Formula Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. No, or nitric oxide has two atoms: So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead.. Nitric Oxide Electron Configuration.
From stock.adobe.com
Nitric oxide, NO, molecule model and chemical formula. Nitrogen oxide Nitric Oxide Electron Configuration So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. The no+ cation, which is formed by. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement.. Nitric Oxide Electron Configuration.
From healthjade.com
Nitric oxide, what does nitric oxide do? Nitric oxide supplements Nitric Oxide Electron Configuration The no+ cation, which is formed by. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Therefore, total valence electrons in no= 5+6 = 11. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one. Nitric Oxide Electron Configuration.
From chemistry.stackexchange.com
energy Molecular orbital diagram for nitrogen monoxide, the nitrosyl Nitric Oxide Electron Configuration Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Here is the mo diagram for nitric oxide: No, or nitric oxide has two atoms: So, the molecular. Nitric Oxide Electron Configuration.
From www.youtube.com
Molecular Orbital Diagram for Nitric Oxide Heteronuclear molecule YouTube Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. No, or nitric oxide has two atoms: Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Therefore, total valence electrons in no= 5+6 = 11. Here is the mo diagram for nitric oxide: The location of the. Nitric Oxide Electron Configuration.
From chemistry.stackexchange.com
chemistry What is "dot" sign in •NO? Chemistry Stack Exchange Nitric Oxide Electron Configuration The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. The no+ cation, which is formed by. Nitrogen has five valence electrons. Nitric Oxide Electron Configuration.
From www.alamy.com
Nitric Oxide, NO, molecule model, chemical formula. Nitrogen oxide Nitric Oxide Electron Configuration The no+ cation, which is formed by. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Here is the mo diagram for nitric oxide: Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Therefore, total valence electrons in no= 5+6 = 11. One n atom, and. Nitric Oxide Electron Configuration.
From ar.inspiredpencil.com
Nitrous Oxide Nitric Oxide Electron Configuration Here is the mo diagram for nitric oxide: Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. The no+ cation, which is formed by. Therefore, total valence electrons in no= 5+6 = 11. One n atom, and one o atom. No, or nitric oxide. Nitric Oxide Electron Configuration.
From chemistry.stackexchange.com
bond Nitric Oxide Dimerization Chemistry Stack Exchange Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. No, or nitric oxide has two atoms: Therefore, total valence electrons in no= 5+6 = 11. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n.. Nitric Oxide Electron Configuration.
From www.youtube.com
NO Lewis Structure How to Draw the Lewis Structure for NO (Nitric Nitric Oxide Electron Configuration So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Therefore, total valence electrons in no= 5+6 = 11. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. Here is the mo diagram. Nitric Oxide Electron Configuration.
From diagramweb.net
Nitric Oxide Molecular Orbital Diagram Nitric Oxide Electron Configuration Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. One n atom, and one o atom. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead.. Nitric Oxide Electron Configuration.
From quizlet.com
Consider the Lewis structure for the nitric acid molecule, H Quizlet Nitric Oxide Electron Configuration One n atom, and one o atom. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitrogen has five valence electrons. Nitric Oxide Electron Configuration.
From valenceelectrons.com
How Many Valence Electrons Does HNO3 (Nitric acid) Have? Nitric Oxide Electron Configuration The no+ cation, which is formed by. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. So, the molecular electron configuration would be written in a similar manner as. Nitric Oxide Electron Configuration.
From pediaa.com
Difference Between Nitric Oxide and Nitrous Oxide Definition Nitric Oxide Electron Configuration One n atom, and one o atom. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. So, the molecular electron configuration would be written in a similar. Nitric Oxide Electron Configuration.
From www.researchgate.net
Nitric oxide synthesis and biological functions. (A) In normoxia Nitric Oxide Electron Configuration Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. One n atom, and one o atom. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitric oxide has a unique electronic configuration,. Nitric Oxide Electron Configuration.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. Therefore, total valence electrons in no= 5+6 = 11. No, or nitric oxide. Nitric Oxide Electron Configuration.
From phys.org
Promoting nitric oxide electroreduction to ammonia over electronrich Nitric Oxide Electron Configuration So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. No, or nitric oxide has two atoms: Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons.. Nitric Oxide Electron Configuration.
From rtmagazine.com
Impact of Cylinderfree Inhaled Nitric Oxide Delivery RT Nitric Oxide Electron Configuration One n atom, and one o atom. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons.. Nitric Oxide Electron Configuration.
From www.dreamstime.com
Nitric Oxide NO Free Radical and Signaling Molecule. Skeletal Formula Nitric Oxide Electron Configuration Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. One n atom, and one o atom. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results. Nitric Oxide Electron Configuration.
From exosblltn.blob.core.windows.net
Zinc Oxide Electron Configuration at Cherry Lee blog Nitric Oxide Electron Configuration Here is the mo diagram for nitric oxide: One n atom, and one o atom. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. No, or nitric. Nitric Oxide Electron Configuration.
From greymattersjournal.org
The Radical Role of Nitric Oxide in Learning Nitric Oxide Electron Configuration The no+ cation, which is formed by. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. No, or nitric oxide has two atoms: Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitrogen has. Nitric Oxide Electron Configuration.
From sciencetrends.com
NO Lewis Dot Structure Science Trends Nitric Oxide Electron Configuration No, or nitric oxide has two atoms: Here is the mo diagram for nitric oxide: Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals. Nitric Oxide Electron Configuration.
From chemistry.stackexchange.com
chemistry Structure of nitric oxide Chemistry Stack Exchange Nitric Oxide Electron Configuration The no+ cation, which is formed by. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. No, or nitric oxide has two atoms: One n atom, and one o atom. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p. Nitric Oxide Electron Configuration.
From brainly.in
The clectronic configuration of oxygen is 2, b) Illustrate the Nitric Oxide Electron Configuration Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. No, or nitric oxide has two atoms: One n atom, and one o atom. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. So, the molecular electron configuration would. Nitric Oxide Electron Configuration.
From 2p3lss.blogspot.com
2P3 LSS 2P333 Nitric Oxide Nitric Oxide Electron Configuration The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Here is the mo diagram for nitric oxide: So, the molecular electron configuration would be written in a. Nitric Oxide Electron Configuration.
From exosblltn.blob.core.windows.net
Zinc Oxide Electron Configuration at Cherry Lee blog Nitric Oxide Electron Configuration No, or nitric oxide has two atoms: Here is the mo diagram for nitric oxide: Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. The no+ cation, which is formed by. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results. Nitric Oxide Electron Configuration.
From www.dreamstime.com
3D Image of Nitric Oxide Skeletal Formula Stock Illustration Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. So, the molecular. Nitric Oxide Electron Configuration.
From valenceelectrons.com
How to Find the Valence Electrons for HNO3 (Nitric acid)? Nitric Oxide Electron Configuration Here is the mo diagram for nitric oxide: Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. No, or nitric oxide has two atoms: The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather. Nitric Oxide Electron Configuration.
From www.pnas.org
Nitric oxide synthase domain interfaces regulate electron transfer and Nitric Oxide Electron Configuration The no+ cation, which is formed by. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. The location of the unpaired electron in the π* orbital (figure \(\pageindex{6}\)a) results in a bond order of 2.5 rather than the triple bond observed for n. Therefore, total valence electrons in no= 5+6 = 11.. Nitric Oxide Electron Configuration.
From exosblltn.blob.core.windows.net
Zinc Oxide Electron Configuration at Cherry Lee blog Nitric Oxide Electron Configuration No, or nitric oxide has two atoms: Therefore, total valence electrons in no= 5+6 = 11. Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron,. Nitric Oxide Electron Configuration.
From leonidaskruwsandoval.blogspot.com
Orbital Diagram for Nitrogen LeonidaskruwSandoval Nitric Oxide Electron Configuration Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitrogen has five valence electrons in its outermost shell and oxygen has six valence electrons. Here is the mo diagram for nitric oxide: Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is. Nitric Oxide Electron Configuration.
From www.alamy.com
Nitric oxide, NO, molecule model and chemical formula. Nitrogen oxide Nitric Oxide Electron Configuration Here is the mo diagram for nitric oxide: The no+ cation, which is formed by. One n atom, and one o atom. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. Nitrogen has five valence electrons in its outermost shell and oxygen has six. Nitric Oxide Electron Configuration.
From www.mooramo.com
Ions of Transition Elements Mooramo Nitric Oxide Electron Configuration So, the molecular electron configuration would be written in a similar manner as the atomic counterpart, but using molecular orbitals instead. The no+ cation, which is formed by. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p 3 electron arrangement. Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and. Nitric Oxide Electron Configuration.
From drjockers.com
Nitric Oxide Benefits and How to Increase Levels Nitric Oxide Electron Configuration Nitric oxide (figure \(\pageindex{6}\)b) is electronically equivalent to dinitrogen (n 2) plus an electron, and as a consequence it is paramagnetic with one unpaired electron. The no+ cation, which is formed by. No, or nitric oxide has two atoms: One n atom, and one o atom. Nitric oxide has a unique electronic configuration, with a 1s 2 2s 2 2p. Nitric Oxide Electron Configuration.