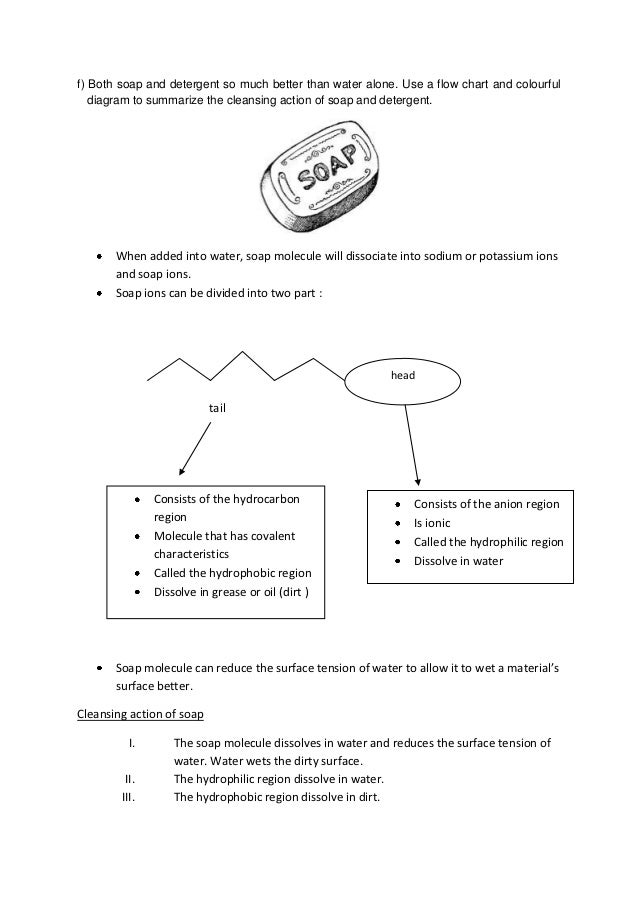
Soap And Detergent Molecules Have A Long . If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soaps and detergents are made from long molecules that contain a head and tail. Click here to learn about the differences between soaps and detergents. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. These molecules are called surfactants; Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Anionic detergents are detergent molecules that contain negatively charged heads. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. The diagram below represents a surfactant molecule.
from www.slideshare.net
The diagram below represents a surfactant molecule. Click here to learn about the differences between soaps and detergents. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps and detergents are made from long molecules that contain a head and tail. These molecules are called surfactants; Anionic detergents are detergent molecules that contain negatively charged heads.
Soap and detergent ( chemistry folio form 5 )
Soap And Detergent Molecules Have A Long Anionic detergents are detergent molecules that contain negatively charged heads. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. The diagram below represents a surfactant molecule. Anionic detergents are detergent molecules that contain negatively charged heads. Soaps and detergents are made from long molecules that contain a head and tail. Click here to learn about the differences between soaps and detergents. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. These molecules are called surfactants;
From www.slideshare.net
Soap and detergents Soap And Detergent Molecules Have A Long Anionic detergents are detergent molecules that contain negatively charged heads. The diagram below represents a surfactant molecule. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. These molecules are called surfactants; Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soap and detergent molecules have a long,. Soap And Detergent Molecules Have A Long.
From nearbayou.com
Soap Vs Detergent What is the difference? NearBayou Soap And Detergent Molecules Have A Long If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Click here to learn about the differences between soaps and detergents. These molecules are called surfactants; Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Soaps and detergents are made from long. Soap And Detergent Molecules Have A Long.
From www.slideshare.net
Soap and detergent ( chemistry folio form 5 ) Soap And Detergent Molecules Have A Long Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. These molecules are called surfactants; The diagram below represents a surfactant molecule. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Anionic detergents are detergent molecules that contain negatively charged heads. Soaps are. Soap And Detergent Molecules Have A Long.
From cosmosmagazine.com
The chemistry of soap Soap And Detergent Molecules Have A Long Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. These molecules are called surfactants; Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Anionic detergents are detergent molecules that contain negatively charged heads. Soaps and detergents are made from. Soap And Detergent Molecules Have A Long.
From www.numerade.com
SOLVED list the differences between soap and detergent. describe why Soap And Detergent Molecules Have A Long Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Detergents are. Soap And Detergent Molecules Have A Long.
From www.slideshare.net
How do soaps work Soap And Detergent Molecules Have A Long Click here to learn about the differences between soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. The diagram below represents a surfactant molecule. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps and detergents are made from long molecules that contain a. Soap And Detergent Molecules Have A Long.
From childhealthpolicy.vumc.org
🐈 Soaps and detergents are. What are Soaps and Detergents?. 20221102 Soap And Detergent Molecules Have A Long The diagram below represents a surfactant molecule. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps and detergents are made from long molecules that contain a head and tail. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Click here to learn about the differences between soaps and detergents. These. Soap And Detergent Molecules Have A Long.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap And Detergent Molecules Have A Long Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Click here to learn about the differences between soaps and detergents. Anionic detergents are detergent molecules that contain negatively charged heads. These molecules are called surfactants; Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. If the ph of a soap solution. Soap And Detergent Molecules Have A Long.
From loeploscy.blob.core.windows.net
Are Detergents Polar Or Nonpolar at John Cline blog Soap And Detergent Molecules Have A Long Click here to learn about the differences between soaps and detergents. The diagram below represents a surfactant molecule. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps are sodium or potassium salts of fatty acids. Soap And Detergent Molecules Have A Long.
From www.numerade.com
SOLVEDSoap molecules have two portions with very different properties Soap And Detergent Molecules Have A Long Soaps and detergents are made from long molecules that contain a head and tail. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. The diagram below represents a surfactant molecule. Click here to learn about the differences between soaps and detergents. Soap and detergent molecules have a long, hydrophobic tail. Soap And Detergent Molecules Have A Long.
From www.slideserve.com
PPT Chapter 30 Detergents PowerPoint Presentation, free download ID Soap And Detergent Molecules Have A Long Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. These molecules are called surfactants; The diagram below represents a surfactant molecule.. Soap And Detergent Molecules Have A Long.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap And Detergent Molecules Have A Long Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. The diagram below represents a surfactant molecule. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. These molecules are called. Soap And Detergent Molecules Have A Long.
From www.pcaplindia.com
Prakash chemicals agencies serving many companies in the field of soap Soap And Detergent Molecules Have A Long Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. The diagram below represents a surfactant molecule. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps and detergents are made from long molecules that contain a head and tail.. Soap And Detergent Molecules Have A Long.
From dxoxxwrfb.blob.core.windows.net
Difference Between Dish Soap And Detergent at Andres Hayton blog Soap And Detergent Molecules Have A Long Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Click here to learn about the differences between soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate. Soap And Detergent Molecules Have A Long.
From www.doubtnut.com
Both soap and detergent are some type of salts. What is the difference Soap And Detergent Molecules Have A Long Anionic detergents are detergent molecules that contain negatively charged heads. The diagram below represents a surfactant molecule. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps are sodium. Soap And Detergent Molecules Have A Long.
From www.numerade.com
SOLVEDSoap molecules have a hydrophobic portion and yet they dissolve Soap And Detergent Molecules Have A Long If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. These molecules are called surfactants; Soaps and detergents are made from long molecules that contain a head and tail. Anionic detergents are detergent molecules that contain negatively charged heads. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids.. Soap And Detergent Molecules Have A Long.
From www.tutorix.com
Detergents and Surface Tension Soap And Detergent Molecules Have A Long These molecules are called surfactants; Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Click here to learn about the differences between soaps and detergents. The diagram below represents a surfactant molecule. Anionic detergents are detergent molecules that contain. Soap And Detergent Molecules Have A Long.
From slideplayer.com
I like nonsense, it wakes up the brain cells ppt download Soap And Detergent Molecules Have A Long Soaps and detergents are made from long molecules that contain a head and tail. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Click here to learn about the differences between soaps and detergents. Anionic detergents are detergent molecules that contain negatively charged heads. Detergents are sodium or potassium salts. Soap And Detergent Molecules Have A Long.
From www.teachoo.com
[MCQ] In the soap micelles (a) the ionic end of soap is on the surface Soap And Detergent Molecules Have A Long Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Anionic detergents are detergent molecules that contain negatively charged heads. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. The. Soap And Detergent Molecules Have A Long.
From www.themacbath.com
Back to Basics What Is Soap and How Does It Work? — The MacBath Soap And Detergent Molecules Have A Long If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Detergents are. Soap And Detergent Molecules Have A Long.
From stock.adobe.com
Micelle formation in oil and detergent solution water, h2o Soap And Detergent Molecules Have A Long Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Click here to learn about the differences between soaps and detergents. These molecules are called surfactants; Soaps and detergents are made from long molecules that contain a head and tail. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soaps are sodium. Soap And Detergent Molecules Have A Long.
From top10gears.com
18 Different Types of Laundry Detergents Soap And Detergent Molecules Have A Long Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. The diagram below represents a surfactant molecule. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. These molecules are called surfactants; Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. If the ph. Soap And Detergent Molecules Have A Long.
From makerscientist.com
Emulsions Magical Mixtures of oil and water? Maker Scientist Soap And Detergent Molecules Have A Long Click here to learn about the differences between soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. The diagram below represents a surfactant molecule. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soaps and detergents are made from long molecules. Soap And Detergent Molecules Have A Long.
From slideplayer.com
Micelles to the Rescue How Soap Transports Debris ppt download Soap And Detergent Molecules Have A Long The diagram below represents a surfactant molecule. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soap and detergent molecules. Soap And Detergent Molecules Have A Long.
From www.slideshare.net
Soap and detergent Soap And Detergent Molecules Have A Long The diagram below represents a surfactant molecule. These molecules are called surfactants; Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Soaps and detergents are made from long molecules that contain a head and tail. Click here to learn about the differences between soaps and detergents. Soaps are sodium or potassium salts of fatty. Soap And Detergent Molecules Have A Long.
From www.aplustopper.com
Explain the Cleansing Action Of Soaps and Detergents A Plus Topper Soap And Detergent Molecules Have A Long Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Anionic detergents are detergent molecules that contain negatively. Soap And Detergent Molecules Have A Long.
From wowscience.co.uk
The Science of Handwashing WowScience Science games and activities Soap And Detergent Molecules Have A Long Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Soaps and detergents are made from long molecules that contain a head and tail. Click here to learn about the differences between soaps and detergents. The diagram below represents a. Soap And Detergent Molecules Have A Long.
From www.slideshare.net
Soap vs detergents Soap And Detergent Molecules Have A Long Click here to learn about the differences between soaps and detergents. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. If the ph of. Soap And Detergent Molecules Have A Long.
From www.researchgate.net
The detergent mechanism by BioS Download Scientific Diagram Soap And Detergent Molecules Have A Long Soaps and detergents are made from long molecules that contain a head and tail. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Anionic detergents are detergent molecules that contain negatively charged heads. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. The diagram below. Soap And Detergent Molecules Have A Long.
From www.curioustem.org
CuriouSTEM The Magic Behind Soap Bubbles Soap And Detergent Molecules Have A Long Soaps and detergents are made from long molecules that contain a head and tail. Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. These molecules are called surfactants; Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Solutions of alkali metal soaps are slightly alkaline. Soap And Detergent Molecules Have A Long.
From www.coursehero.com
[Solved] Draw the chemical structure of a soap and a detergent (choose Soap And Detergent Molecules Have A Long Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. Anionic detergents are detergent molecules that contain negatively charged heads. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soap and detergent molecules have. Soap And Detergent Molecules Have A Long.
From byjus.com
Cleansing Action Of Soaps And Detergents Micelle Formation & Action Soap And Detergent Molecules Have A Long Anionic detergents are detergent molecules that contain negatively charged heads. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Detergents are sodium or potassium salts of aliphatic or aromatic sulphonic acids. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. If the ph of a. Soap And Detergent Molecules Have A Long.
From www.thoughtco.com
How Soap Works Soap And Detergent Molecules Have A Long Soaps and detergents are made from long molecules that contain a head and tail. Solutions of alkali metal soaps are slightly alkaline (ph 8 to 9) due to hydrolysis. Click here to learn about the differences between soaps and detergents. Soaps are sodium or potassium salts of fatty acids which are formed by hydrolysis of fats and oils. The diagram. Soap And Detergent Molecules Have A Long.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap And Detergent Molecules Have A Long Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they are sometimes referred to as bridge. Soaps and detergents are made from long molecules that contain a head and tail. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. Soaps are sodium or potassium salts. Soap And Detergent Molecules Have A Long.
From dokumen.tips
(PDF) Soap and detergent.pdf DOKUMEN.TIPS Soap And Detergent Molecules Have A Long Anionic detergents are detergent molecules that contain negatively charged heads. Click here to learn about the differences between soaps and detergents. If the ph of a soap solution is lowered by acidic contaminants, insoluble fatty acids precipitate and form a. These molecules are called surfactants; Soap and detergent molecules have a long, hydrophobic tail and a polar, hydrophilic head. they. Soap And Detergent Molecules Have A Long.