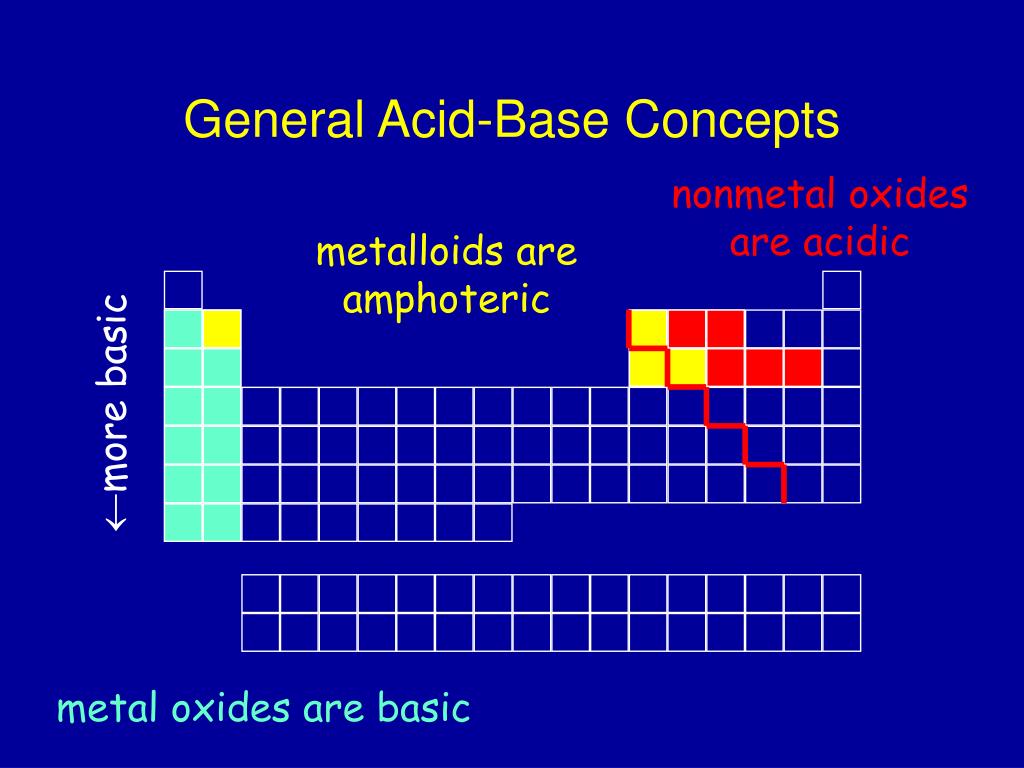
Are Metalloids Acidic Or Basic . Elements to the left are metals and nonmetals are. These elements have the ability to form metallic alloys. Metallic oxides are basic, ionic. Anions, oxyanions in aqueous solution The oxidation number of an element in this group can. Metalloids are known to form amphoteric or weakly acidic oxides. This periodic table shows the three different groups of elements. The metalloids are boron, silicon,. Nonmetallic oxides are acidic, compounds. The metalloid group separates the metals from the nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases.
from www.slideserve.com
Nonmetallic oxides are acidic, compounds. The metalloids are boron, silicon,. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Anions, oxyanions in aqueous solution A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloid group separates the metals from the nonmetals. Metallic oxides are basic, ionic. This periodic table shows the three different groups of elements. These elements have the ability to form metallic alloys. Elements to the left are metals and nonmetals are.
PPT AcidBase Concepts PowerPoint Presentation, free download ID581017
Are Metalloids Acidic Or Basic Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are known to form amphoteric or weakly acidic oxides. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Nonmetallic oxides are acidic, compounds. Elements to the left are metals and nonmetals are. Metallic oxides are basic, ionic. The metalloid group separates the metals from the nonmetals. This periodic table shows the three different groups of elements. The oxidation number of an element in this group can. These elements have the ability to form metallic alloys. Anions, oxyanions in aqueous solution
From slideplayer.com
CHEM 120 WEEK 12 LECTURES WEEK 3) ppt download Are Metalloids Acidic Or Basic The metalloids are boron, silicon,. This periodic table shows the three different groups of elements. Nonmetallic oxides are acidic, compounds. Metallic oxides are basic, ionic. Elements to the left are metals and nonmetals are. Anions, oxyanions in aqueous solution The oxidation number of an element in this group can. These elements have the ability to form metallic alloys. A series. Are Metalloids Acidic Or Basic.
From pediabay.com
Metalloids of the Periodic Table Pediabay Are Metalloids Acidic Or Basic Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Elements to the left are metals and nonmetals are. The oxidation number of an element in this group can. The metalloid group separates the metals from the nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT AcidBase Concepts PowerPoint Presentation, free download ID581017 Are Metalloids Acidic Or Basic This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. The oxidation number of an element in this group can. Metallic oxides are basic, ionic. Elements to the left are metals and nonmetals are. Nonmetallic oxides are acidic, compounds. These elements have the ability to form metallic alloys. Metalloids are known. Are Metalloids Acidic Or Basic.
From sciencenotes.org
List of Metalloids or Semimetals Are Metalloids Acidic Or Basic Nonmetallic oxides are acidic, compounds. Metallic oxides are basic, ionic. Anions, oxyanions in aqueous solution The oxidation number of an element in this group can. The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are known to form amphoteric or weakly acidic oxides. These elements. Are Metalloids Acidic Or Basic.
From www.pinterest.com
5 Examples of Metals, Metalloids, and Nonmetals Metals nonmetals metalloids, Sixth grade Are Metalloids Acidic Or Basic Elements to the left are metals and nonmetals are. Metalloids are known to form amphoteric or weakly acidic oxides. Metallic oxides are basic, ionic. Nonmetallic oxides are acidic, compounds. This periodic table shows the three different groups of elements. The metalloid group separates the metals from the nonmetals. Metalloids often form amphoteric oxides, meaning their oxides can react with both. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT 7.6 Metals, Nonmetals, and Metalloids PowerPoint Presentation, free download ID6920412 Are Metalloids Acidic Or Basic Metalloids are known to form amphoteric or weakly acidic oxides. Anions, oxyanions in aqueous solution A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloid group separates the metals from the nonmetals. Metallic oxides are basic, ionic. Nonmetallic oxides are acidic, compounds. Metalloids often form amphoteric oxides, meaning their oxides. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT 7.6 Metals, Nonmetals, and Metalloids PowerPoint Presentation ID3646818 Are Metalloids Acidic Or Basic The metalloid group separates the metals from the nonmetals. Elements to the left are metals and nonmetals are. Metallic oxides are basic, ionic. Anions, oxyanions in aqueous solution The metalloids are boron, silicon,. These elements have the ability to form metallic alloys. Nonmetallic oxides are acidic, compounds. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids. Are Metalloids Acidic Or Basic.
From homedeso.vercel.app
Metals And Metalloids On Periodic Table Are Metalloids Acidic Or Basic Elements to the left are metals and nonmetals are. Nonmetallic oxides are acidic, compounds. The metalloids are boron, silicon,. The oxidation number of an element in this group can. These elements have the ability to form metallic alloys. The metalloid group separates the metals from the nonmetals. This periodic table shows the three different groups of elements. Metalloids are known. Are Metalloids Acidic Or Basic.
From quizlet.com
Metals, Metalloids, and NonMetals Properties & Where They Are Diagram Quizlet Are Metalloids Acidic Or Basic Anions, oxyanions in aqueous solution The oxidation number of an element in this group can. Metallic oxides are basic, ionic. These elements have the ability to form metallic alloys. The metalloid group separates the metals from the nonmetals. Metalloids are known to form amphoteric or weakly acidic oxides. Nonmetallic oxides are acidic, compounds. Elements to the left are metals and. Are Metalloids Acidic Or Basic.
From dl-uk.apowersoft.com
Periodic Table Of Elements Metals Nonmetals Metalloids Printable Are Metalloids Acidic Or Basic A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are known to form amphoteric or weakly acidic oxides. Metallic oxides are basic, ionic. These elements have the ability to form metallic alloys. The metalloids are boron, silicon,. Anions, oxyanions in aqueous solution Metalloids often form amphoteric oxides, meaning their oxides. Are Metalloids Acidic Or Basic.
From www.adda247.com
What are metalloids? Definition, Properties and Example Are Metalloids Acidic Or Basic A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. These elements have the ability to form metallic alloys. The metalloids are boron, silicon,. Nonmetallic oxides are acidic, compounds. Metallic oxides are basic, ionic. Metalloids are known to form amphoteric or weakly acidic oxides. Metalloids often form amphoteric oxides, meaning their oxides. Are Metalloids Acidic Or Basic.
From slideplayer.com
Ch 2 Matter and Change. ppt download Are Metalloids Acidic Or Basic The oxidation number of an element in this group can. Nonmetallic oxides are acidic, compounds. Elements to the left are metals and nonmetals are. The metalloid group separates the metals from the nonmetals. Anions, oxyanions in aqueous solution The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic. Are Metalloids Acidic Or Basic.
From slideplayer.com
Periodic Relationships Among the Elements ppt download Are Metalloids Acidic Or Basic This periodic table shows the three different groups of elements. Anions, oxyanions in aqueous solution The metalloids are boron, silicon,. Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloid group separates the metals from the nonmetals. Nonmetallic oxides are acidic,. Are Metalloids Acidic Or Basic.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts NewtonDesk Are Metalloids Acidic Or Basic A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Metallic oxides are basic, ionic. The metalloids are boron, silicon,. This periodic table shows the three different groups of elements. The oxidation number of an element in. Are Metalloids Acidic Or Basic.
From www.xometry.com
Your Guide to Understanding Metalloids Are Metalloids Acidic Or Basic Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metallic oxides are basic, ionic. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Nonmetallic oxides are acidic, compounds. The metalloid group separates the metals from the. Are Metalloids Acidic Or Basic.
From knordslearning.com
Periodic Table Metals, Nonmetals & Metalloids (With Images) Are Metalloids Acidic Or Basic Nonmetallic oxides are acidic, compounds. This periodic table shows the three different groups of elements. Metallic oxides are basic, ionic. Anions, oxyanions in aqueous solution Metalloids are known to form amphoteric or weakly acidic oxides. These elements have the ability to form metallic alloys. The oxidation number of an element in this group can. A series of six elements called. Are Metalloids Acidic Or Basic.
From www.baamboozle.com
Metals, Nonmetals, and Metalloids Baamboozle Are Metalloids Acidic Or Basic The oxidation number of an element in this group can. Elements to the left are metals and nonmetals are. The metalloids are boron, silicon,. Metalloids are known to form amphoteric or weakly acidic oxides. Metallic oxides are basic, ionic. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Anions, oxyanions in. Are Metalloids Acidic Or Basic.
From kgrayschs.weebly.com
Ch 5, 6 Electrons in the Atom and The Periodic Table Are Metalloids Acidic Or Basic Metallic oxides are basic, ionic. Metalloids are known to form amphoteric or weakly acidic oxides. Elements to the left are metals and nonmetals are. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. The metalloid group separates the metals from the nonmetals. These elements have the ability to form metallic alloys. Nonmetallic oxides are. Are Metalloids Acidic Or Basic.
From slideplayer.com
Periodic Properties of the Elements ppt download Are Metalloids Acidic Or Basic Anions, oxyanions in aqueous solution Nonmetallic oxides are acidic, compounds. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. This periodic table shows the three different groups of elements. The metalloid group separates the metals from. Are Metalloids Acidic Or Basic.
From pediabay.com
Periodic Table Metals, Nonmetals & Metalloids Pediabay Are Metalloids Acidic Or Basic Metalloids are known to form amphoteric or weakly acidic oxides. Nonmetallic oxides are acidic, compounds. The metalloid group separates the metals from the nonmetals. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The oxidation number of an element in this group can. Elements to the left are metals and nonmetals. Are Metalloids Acidic Or Basic.
From www.teachoo.com
Metals, Non Metals and Metalloids Meaning & Difference Teachoo Are Metalloids Acidic Or Basic Nonmetallic oxides are acidic, compounds. The metalloids are boron, silicon,. The metalloid group separates the metals from the nonmetals. Elements to the left are metals and nonmetals are. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. This periodic table shows the three different groups of elements. These elements have the. Are Metalloids Acidic Or Basic.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Are Metalloids Acidic Or Basic A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are known to form amphoteric or weakly acidic oxides. This periodic table shows the three different groups of elements. Elements to the left are metals and nonmetals are. The metalloid group separates the metals from the nonmetals. These elements have the. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT Atoms and Elements PowerPoint Presentation, free download ID279571 Are Metalloids Acidic Or Basic These elements have the ability to form metallic alloys. Nonmetallic oxides are acidic, compounds. The metalloid group separates the metals from the nonmetals. Anions, oxyanions in aqueous solution The metalloids are boron, silicon,. This periodic table shows the three different groups of elements. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Metallic oxides. Are Metalloids Acidic Or Basic.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Acidic Or Basic The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Anions, oxyanions in aqueous solution Elements to the left are metals and nonmetals are. This periodic table shows the three different groups of elements. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT THE PERIODIC TABLE PowerPoint Presentation, free download ID9370502 Are Metalloids Acidic Or Basic Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. The oxidation number of an element in this group can. Metalloids are known to form amphoteric or weakly acidic oxides. The metalloids are boron, silicon,. Metallic oxides are basic, ionic. Nonmetallic oxides are acidic, compounds. A series of six elements called the metalloids separate the. Are Metalloids Acidic Or Basic.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Are Metalloids Acidic Or Basic The oxidation number of an element in this group can. These elements have the ability to form metallic alloys. The metalloid group separates the metals from the nonmetals. Anions, oxyanions in aqueous solution Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. Metallic oxides are basic, ionic. Nonmetallic oxides are acidic, compounds. The metalloids. Are Metalloids Acidic Or Basic.
From sciencenotes.org
List of Metalloids or Semimetals Are Metalloids Acidic Or Basic The oxidation number of an element in this group can. Elements to the left are metals and nonmetals are. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metallic oxides are basic, ionic. Metalloids are known. Are Metalloids Acidic Or Basic.
From slideplayer.com
Metals, Nonmetals, and Metalloids ppt download Are Metalloids Acidic Or Basic Elements to the left are metals and nonmetals are. The metalloid group separates the metals from the nonmetals. Metalloids are known to form amphoteric or weakly acidic oxides. Nonmetallic oxides are acidic, compounds. The metalloids are boron, silicon,. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. These elements have the. Are Metalloids Acidic Or Basic.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1587804 Are Metalloids Acidic Or Basic Nonmetallic oxides are acidic, compounds. The metalloids are boron, silicon,. Metalloids are known to form amphoteric or weakly acidic oxides. Anions, oxyanions in aqueous solution The metalloid group separates the metals from the nonmetals. Metallic oxides are basic, ionic. These elements have the ability to form metallic alloys. This periodic table shows the three different groups of elements. Elements to. Are Metalloids Acidic Or Basic.
From www.periodictableprintable.com
Metal And Metalloids Periodic Table 2023 Periodic Table Printable Are Metalloids Acidic Or Basic This periodic table shows the three different groups of elements. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Metalloids are known to form amphoteric or weakly acidic oxides. Anions, oxyanions in aqueous solution The metalloid. Are Metalloids Acidic Or Basic.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Are Metalloids Acidic Or Basic Metallic oxides are basic, ionic. This periodic table shows the three different groups of elements. These elements have the ability to form metallic alloys. A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloid group separates the metals from the nonmetals. The oxidation number of an element in this group. Are Metalloids Acidic Or Basic.
From www.animalia-life.club
Periodic Table With Metals Nonmetals And Metalloids Are Metalloids Acidic Or Basic The metalloids are boron, silicon,. Anions, oxyanions in aqueous solution Metallic oxides are basic, ionic. Metalloids are known to form amphoteric or weakly acidic oxides. The metalloid group separates the metals from the nonmetals. Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. These elements have the ability to form metallic alloys. Elements to. Are Metalloids Acidic Or Basic.
From ar.inspiredpencil.com
Periodic Table Metals Nonmetals Metalloids Labeled Are Metalloids Acidic Or Basic A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. Elements to the left are metals and nonmetals are. These elements have the ability to form metallic alloys. The oxidation number of an element in this group can. Metallic oxides are basic, ionic. Metalloids are known to form amphoteric or weakly acidic. Are Metalloids Acidic Or Basic.
From www.youtube.com
Metalloids & Acidic Oxides & Basic Oxides YouTube Are Metalloids Acidic Or Basic Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. The oxidation number of an element in this group can. Metalloids are known to form amphoteric or weakly acidic oxides. Elements to the left are metals and nonmetals are. The metalloid group separates the metals from the nonmetals. This periodic table shows the three different. Are Metalloids Acidic Or Basic.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Are Metalloids Acidic Or Basic Nonmetallic oxides are acidic, compounds. Anions, oxyanions in aqueous solution Metalloids often form amphoteric oxides, meaning their oxides can react with both acids and bases. These elements have the ability to form metallic alloys. The metalloid group separates the metals from the nonmetals. Metalloids are known to form amphoteric or weakly acidic oxides. Metallic oxides are basic, ionic. The oxidation. Are Metalloids Acidic Or Basic.