Titration Neutralization Formula . You then slowly add a known. Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Perform calculations to determine concentration of unknown acid or base. Hcl + naoh → nacl + h 2 o. The example below demonstrates the.
from www.studocu.com
The example below demonstrates the. Perform calculations to determine concentration of unknown acid or base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. Describe how to perform a titration experiment. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. You then slowly add a known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Hcl + naoh → nacl + h 2 o.
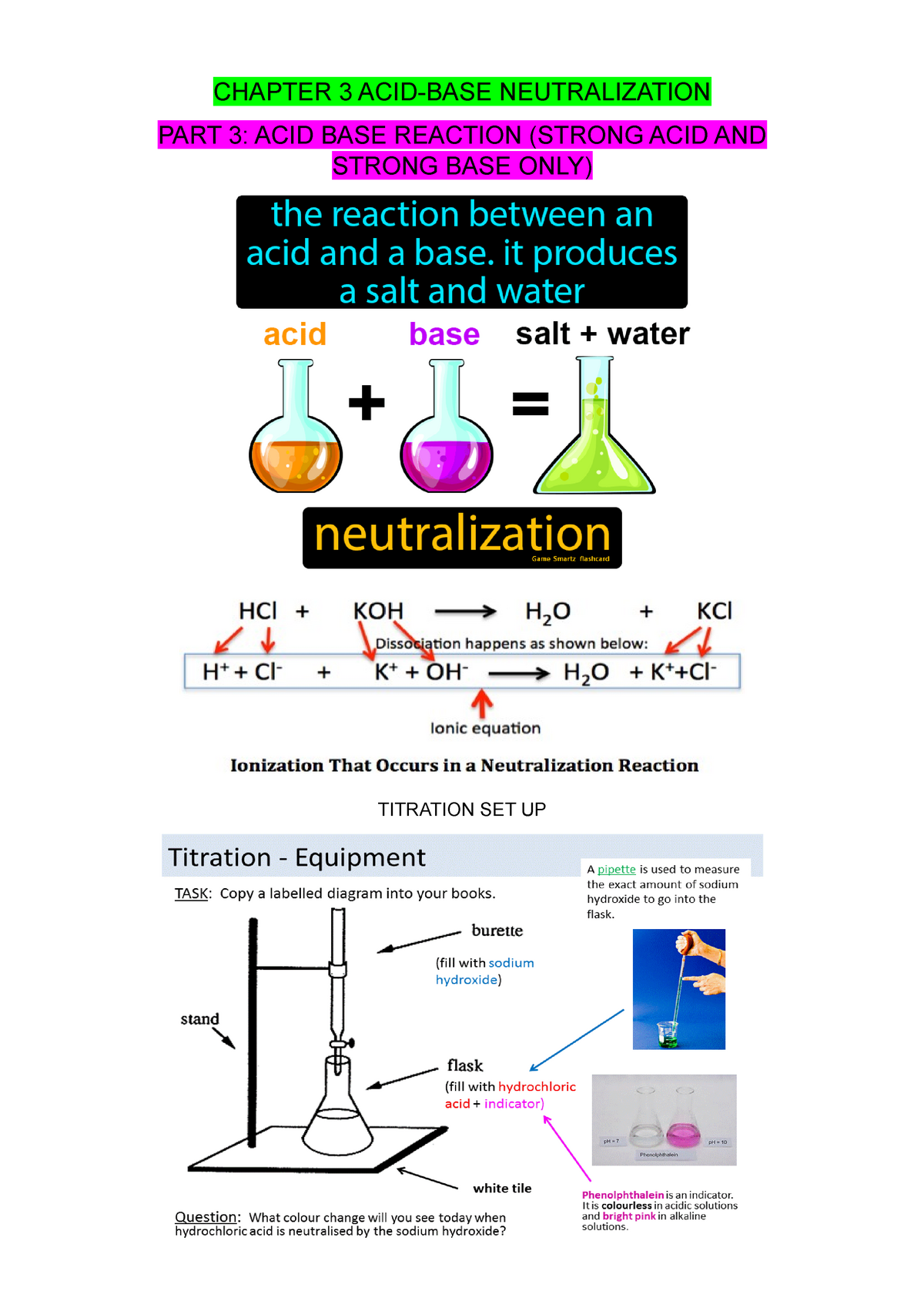
Chapter 3 acid base neutralization part C titration CHAPTER 3 ACIDBASE NEUTRALIZATION PART 3
Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Perform calculations to determine concentration of unknown acid or base. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. You then slowly add a known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Hcl + naoh → nacl + h 2 o.
From exyyylzwb.blob.core.windows.net
Titration In Pharmaceutical Industry at Jamel Edwards blog Titration Neutralization Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. You then slowly add a known. Hcl + naoh → nacl. Titration Neutralization Formula.
From www.slideshare.net
Neutralization titration Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh −. Titration Neutralization Formula.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download ID2145014 Titration Neutralization Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The example below demonstrates the. The above equation works only for. Titration Neutralization Formula.
From joiswenze.blob.core.windows.net
Titration Volume Equation at Shirley Woodard blog Titration Neutralization Formula You then slowly add a known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. Describe how to perform a titration experiment. Perform calculations to determine concentration of unknown acid or base. The following example exercise demonstrates the computation of ph for a titration. Titration Neutralization Formula.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. Perform calculations to determine concentration of unknown acid or base. The example below demonstrates the. You then slowly add a known. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The. Titration Neutralization Formula.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Neutralization Formula Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Perform calculations to determine. Titration Neutralization Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. You then slowly add a known. The example below demonstrates the. Perform calculations to determine concentration of unknown acid or base. Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied. Titration Neutralization Formula.
From www.slideshare.net
Neutralization titration Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Hcl + naoh → nacl + h 2 o. You then slowly add a known. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions. Titration Neutralization Formula.
From slidetodoc.com
Aim What is titration Write the completed neutralization Titration Neutralization Formula In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. Describe how to perform a titration experiment. Perform calculations to determine concentration of unknown acid or base. The following example exercise demonstrates the computation of ph for a titration solution after. Titration Neutralization Formula.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The following example exercise demonstrates the computation of ph for a. Titration Neutralization Formula.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Titration Neutralization Formula The example below demonstrates the. Perform calculations to determine concentration of unknown acid or base. Describe how to perform a titration experiment. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. You then slowly add a known. The above equation works only for neutralizations in which there is a. Titration Neutralization Formula.
From www.slideserve.com
PPT Neutralization Reactions PowerPoint Presentation, free download ID5123724 Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. The example below demonstrates the. You then slowly add a known. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. Describe how to perform a titration experiment. The following example exercise. Titration Neutralization Formula.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Titration Neutralization Formula The example below demonstrates the. Perform calculations to determine concentration of unknown acid or base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of. Titration Neutralization Formula.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Neutralization Formula Perform calculations to determine concentration of unknown acid or base. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h +. Titration Neutralization Formula.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Neutralization Formula In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. You then slowly add a known. Describe how to perform a titration experiment. Perform calculations to determine concentration of unknown acid or base. The example below demonstrates the. Hcl + naoh. Titration Neutralization Formula.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Neutralization Formula Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant. Titration Neutralization Formula.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Neutralization Formula Describe how to perform a titration experiment. Perform calculations to determine concentration of unknown acid or base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. You then slowly add a known. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be. Titration Neutralization Formula.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Chemistry Power Point WITH Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. You then slowly add a known. Describe how to perform a titration experiment. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a balanced neutralization equation, the moles of h + ions supplied by. Titration Neutralization Formula.
From www.youtube.com
Chemistry 18.13 neutralization calculation YouTube Titration Neutralization Formula In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is. Titration Neutralization Formula.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download ID2145014 Titration Neutralization Formula The example below demonstrates the. You then slowly add a known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Perform calculations to determine concentration of unknown acid or base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to. Titration Neutralization Formula.
From www.studocu.com
Chapter 3 acid base neutralization part C titration CHAPTER 3 ACIDBASE NEUTRALIZATION PART 3 Titration Neutralization Formula You then slowly add a known. Perform calculations to determine concentration of unknown acid or base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The example below demonstrates the. Describe how to perform a titration experiment. The above equation. Titration Neutralization Formula.
From www.slideserve.com
PPT Acids and Bases Titration PowerPoint Presentation, free download ID4340726 Titration Neutralization Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the. You then slowly add a known. Hcl + naoh → nacl + h 2 o.. Titration Neutralization Formula.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Hcl + naoh → nacl + h 2 o. The example below demonstrates the. You then slowly add a known. Perform calculations to determine concentration of unknown acid or base. Describe how to perform a titration experiment. In a balanced. Titration Neutralization Formula.
From www.slideshare.net
Neutralization titrations Titration Neutralization Formula You then slowly add a known. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. Hcl + naoh → nacl. Titration Neutralization Formula.
From www.slideserve.com
PPT Chapter 14 Principles of Neutralization Titrations PowerPoint Presentation ID258632 Titration Neutralization Formula The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Hcl + naoh → nacl + h 2 o. You then slowly add a known. Perform calculations to determine concentration of unknown acid or base. The example below demonstrates the. The above equation works only for neutralizations in which there. Titration Neutralization Formula.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Neutralization Formula Describe how to perform a titration experiment. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. Perform calculations to determine. Titration Neutralization Formula.
From www.youtube.com
Acid Base Neutralization Reactions & Net Ionic Equations Chemistry YouTube Titration Neutralization Formula Describe how to perform a titration experiment. Hcl + naoh → nacl + h 2 o. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. The example below demonstrates the. The above equation works only for neutralizations in which there. Titration Neutralization Formula.
From www.youtube.com
212 Exp 5 Neutralization Titration In Aqueous Medium YouTube Titration Neutralization Formula Perform calculations to determine concentration of unknown acid or base. Describe how to perform a titration experiment. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh. Titration Neutralization Formula.
From kladcyhwk.blob.core.windows.net
Titration Formula Pdf at Brandon Rodriguez blog Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. Perform calculations to determine concentration of unknown acid or base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In. Titration Neutralization Formula.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345 Titration Neutralization Formula Perform calculations to determine concentration of unknown acid or base. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions supplied by the base. Hcl. Titration Neutralization Formula.
From www.slideserve.com
PPT Neutralization & Titration PowerPoint Presentation, free download ID2145014 Titration Neutralization Formula The example below demonstrates the. Hcl + naoh → nacl + h 2 o. You then slowly add a known. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the. Titration Neutralization Formula.
From www.slideshare.net
Neutralization titration Titration Neutralization Formula The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Describe how to perform a titration experiment. Hcl + naoh → nacl + h 2 o. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization. Titration Neutralization Formula.
From dxomwdfni.blob.core.windows.net
Titration Ph Formula at Farrah Carleton blog Titration Neutralization Formula Perform calculations to determine concentration of unknown acid or base. The example below demonstrates the. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. In a balanced neutralization equation, the moles of h + ions supplied by the acid will be equal to the moles of oh − ions. Titration Neutralization Formula.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Foundation Titration Neutralization Formula Hcl + naoh → nacl + h 2 o. You then slowly add a known. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The example below demonstrates the.. Titration Neutralization Formula.
From cesdjxor.blob.core.windows.net
Titration Neutralization Equation at Staci Arroyo blog Titration Neutralization Formula Perform calculations to determine concentration of unknown acid or base. Hcl + naoh → nacl + h 2 o. The following example exercise demonstrates the computation of ph for a titration solution after additions of several specified titrant volumes. You then slowly add a known. In a balanced neutralization equation, the moles of h + ions supplied by the acid. Titration Neutralization Formula.