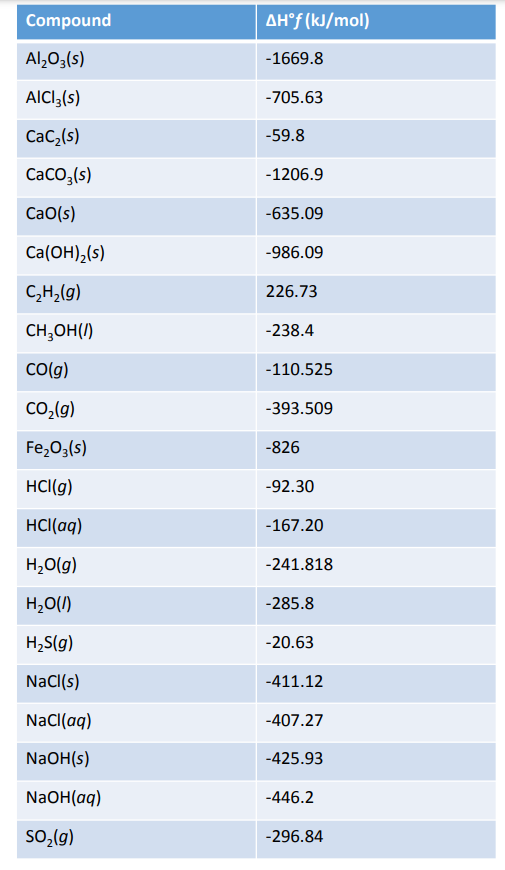
Standard Enthalpy Of Formation Reference Form . the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. the standard enthalpy of formation of any element in its standard state is zero by definition. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, although oxygen can exist as ozone (o 3), atomic. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables.
from www.chegg.com
the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. For example, although oxygen can exist as ozone (o 3), atomic. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation of any element in its standard state is zero by definition.
Solved Using the table of standard enthalpies of formation
Standard Enthalpy Of Formation Reference Form 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. For example, although oxygen can exist as ozone (o 3), atomic. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. the standard enthalpy of formation of any element in its standard state is zero by definition.
From slideplayer.com
Enthalpies of Formation ppt download Standard Enthalpy Of Formation Reference Form the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: a. Standard Enthalpy Of Formation Reference Form.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Reference Form.
From slideplayer.com
Thermodynamics Thermochemistry ppt download Standard Enthalpy Of Formation Reference Form the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of. Standard Enthalpy Of Formation Reference Form.
From www.researchgate.net
Molecular weight, standard enthalpy of formation and thermal Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign. Standard Enthalpy Of Formation Reference Form.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Reference Form definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. the standard enthalpy of formation of. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Reference Form definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. For example, although oxygen can exist as ozone (o 3), atomic. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj the standard enthalpy of formation is a measure of the energy. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation of any element in its standard state is zero by definition. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. the standard enthalpy of formation is given the symbol. Standard Enthalpy Of Formation Reference Form.
From studylib.net
Standard Enthalpy of Formation Standard Enthalpy Of Formation Reference Form a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. . Standard Enthalpy Of Formation Reference Form.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Reference Form the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. . Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Reference Form For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation Reference Form.
From dshfembfeco.blob.core.windows.net
Standard Enthalpy Of Formation So2 at Betty Joseph blog Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol. Standard Enthalpy Of Formation Reference Form.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Reference Form the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when. Standard Enthalpy Of Formation Reference Form.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Reference Form a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. . Standard Enthalpy Of Formation Reference Form.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Reference Form the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. definition and explanation of. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Reference Form For example, although oxygen can exist as ozone (o 3), atomic. the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. the standard enthalpy of formation of any element in its standard state is zero by definition. standard enthalpy of formation (or heat of formation), δh. Standard Enthalpy Of Formation Reference Form.
From www.researchgate.net
Enthalpies of formation (from K 2 LnCl 5 + KCl mixtures) and enthalpies Standard Enthalpy Of Formation Reference Form For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. definition and explanation of the terms standard state and standard enthalpy of formation, with. Standard Enthalpy Of Formation Reference Form.
From www.studocu.com
Enthalpy definitions The Standard Enthalpy of Formation, H f, 298, is Standard Enthalpy Of Formation Reference Form 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. . Standard Enthalpy Of Formation Reference Form.
From www.researchgate.net
The calculated M index and the experimental standard enthalpy of Standard Enthalpy Of Formation Reference Form For example, although oxygen can exist as ozone (o 3), atomic. the standard enthalpy of formation of any element in its standard state is zero by definition. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj For example, the formation of 1 mol ammonia from h 2 and n 2 gases. Standard Enthalpy Of Formation Reference Form.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Standard Enthalpy Of Formation Reference Form standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. . Standard Enthalpy Of Formation Reference Form.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Reference Form a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. For example, although oxygen can exist as ozone (o 3), atomic. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID2567855 Standard Enthalpy Of Formation Reference Form For example, although oxygen can exist as ozone (o 3), atomic. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. definition and explanation of the terms standard state and standard enthalpy of formation, with listing. Standard Enthalpy Of Formation Reference Form.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. For example, although oxygen can exist as ozone (o 3), atomic. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. For example, the formation of 1 mol ammonia. Standard Enthalpy Of Formation Reference Form.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the values of the standard enthalpy of formation in kilojoules per mole for a compound. Standard Enthalpy Of Formation Reference Form.
From www.researchgate.net
(PDF) Standard enthalpies of formation of Li, Na, K, and Cs thiolates Standard Enthalpy Of Formation Reference Form standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. definition and explanation. Standard Enthalpy Of Formation Reference Form.
From www.studocu.com
Standard Enthalpies of Formation & Standard Entropies kJ J ( mol Standard Enthalpy Of Formation Reference Form standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, although oxygen can exist as ozone (o 3), atomic. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, the formation of 1. Standard Enthalpy Of Formation Reference Form.
From www.madebyteachers.com
Standard Molar Enthalpy of Formation Calculations A Chemistry Standard Enthalpy Of Formation Reference Form 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. For example, the formation of 1 mol ammonia from h 2 and. Standard Enthalpy Of Formation Reference Form.
From www.studeersnel.nl
Rstandard enthalpy of formation Table useful Standard Enthalpy of Standard Enthalpy Of Formation Reference Form 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation is given the symbol δfho δ f h o,. Standard Enthalpy Of Formation Reference Form.
From slideplayer.com
Chapter 4 Thermochemistry. ppt download Standard Enthalpy Of Formation Reference Form 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0. Standard Enthalpy Of Formation Reference Form.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript degree sign indicates. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. the standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation Reference Form.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Reference Form the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example, the formation of 1 mol ammonia from h 2 and n 2 gases releases 46.0 kj heat: standard enthalpy of formation (or heat of formation), δh o f , is the enthalpy change when. Standard Enthalpy Of Formation Reference Form.
From slideplayer.com
Thermochemistry Chapter ppt download Standard Enthalpy Of Formation Reference Form definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. a standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from. the standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Reference Form.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Reference Form For example, although oxygen can exist as ozone (o 3), atomic. the values of the standard enthalpy of formation in kilojoules per mole for a compound can be found in reference tables. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Reference Form.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Reference Form For example, although oxygen can exist as ozone (o 3), atomic. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 1.5h 2 (g) + 0.5n 2 (g) ⇆ nh 3 (g) δh o f = 46.0 kj the standard enthalpy of formation of any element. Standard Enthalpy Of Formation Reference Form.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Reference Form 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. the standard enthalpy of formation is given the symbol δfho δ f h o, where the superscript. Standard Enthalpy Of Formation Reference Form.