Fda Guidance Investigational Drug Labeling . 312.6 labeling of an investigational new drug. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. The initial ind submission to the fda will provide the reviewers with the. investigational new drugs or biologics. (a) the immediate package of an. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) the immediate package of an investigational new drug intended for human. § 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies.
from hivinfo.nih.gov
guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. (a) the immediate package of an. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. investigational new drugs or biologics. The initial ind submission to the fda will provide the reviewers with the. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies.
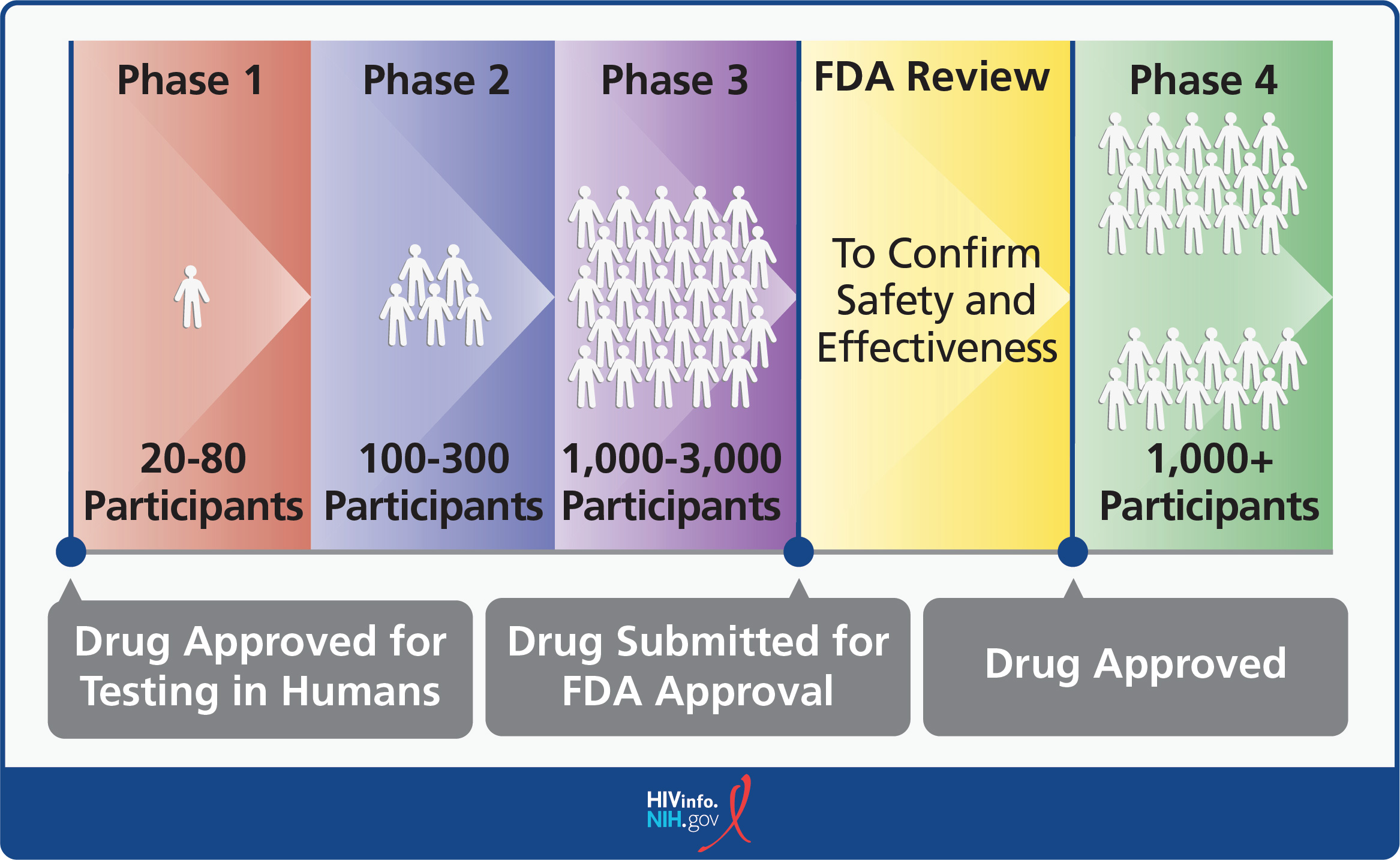
What is an Investigational HIV Drug? NIH
Fda Guidance Investigational Drug Labeling (a) the immediate package of an. investigational new drugs or biologics. (a) the immediate package of an investigational new drug intended for human. The initial ind submission to the fda will provide the reviewers with the. (a) the immediate package of an. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. § 312.6 labeling of an investigational new drug. 312.6 labeling of an investigational new drug.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) this part contains procedures. Fda Guidance Investigational Drug Labeling.
From www.fda.gov
How Do I Use Prescription Drug Labeling FDA Fda Guidance Investigational Drug Labeling The initial ind submission to the fda will provide the reviewers with the. (a) the immediate package of an. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should. Fda Guidance Investigational Drug Labeling.
From dandelionsandthings.blogspot.com
33 The Dispensing Label On An Outpatient Pharmacy Prescription Requires Label Design Ideas 2020 Fda Guidance Investigational Drug Labeling (a) the immediate package of an. (a) the immediate package of an investigational new drug intended for human. 312.6 labeling of an investigational new drug. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. investigational new drugs or biologics.. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. investigational new drugs or biologics. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. this guidance is intended to assist applicants. Fda Guidance Investigational Drug Labeling.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. investigational new. Fda Guidance Investigational Drug Labeling.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Presentation ID3414053 Fda Guidance Investigational Drug Labeling § 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. The initial ind submission to the fda will provide the reviewers with the. investigational new drugs or biologics. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human.. Fda Guidance Investigational Drug Labeling.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) the immediate package of an investigational new drug intended for human. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. investigational new drugs or biologics. guidance. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. § 312.6 labeling of an investigational new drug. (a) this part contains procedures and requirements governing the use of investigational. Fda Guidance Investigational Drug Labeling.
From hivinfo.nih.gov
What is an Investigational HIV Drug? NIH Fda Guidance Investigational Drug Labeling The initial ind submission to the fda will provide the reviewers with the. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. § 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. guidance documents listed below represent. Fda Guidance Investigational Drug Labeling.
From research.chop.edu
Do I need an IND? Fda Guidance Investigational Drug Labeling (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) the immediate package. Fda Guidance Investigational Drug Labeling.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. investigational new drugs or biologics. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. this guidance is intended to assist applicants in complying with the content and format. Fda Guidance Investigational Drug Labeling.
From www.slideshare.net
Fda proposes new guide for drug labeling Fda Guidance Investigational Drug Labeling (a) the immediate package of an. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. 312.6 labeling of an investigational new drug. investigational new drugs or biologics. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the. Fda Guidance Investigational Drug Labeling.
From www.researchgate.net
(PDF) Information Extraction From FDA Drug Labeling to Enhance ProductSpecific Guidance Fda Guidance Investigational Drug Labeling (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) the immediate package of an. investigational new drugs or biologics. The initial ind submission to the fda will provide the reviewers with. Fda Guidance Investigational Drug Labeling.
From www.europeanpharmaceuticalreview.com
FDA releases guidance on pharmaceutical product labelling Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. investigational new drugs or biologics. (a) the immediate package of an investigational new drug intended for human. 312.6 labeling of an investigational new. Fda Guidance Investigational Drug Labeling.
From academicentrepreneurship.pubpub.org
FDA Drug Regulation Investigational New Drug Applications · Academic Entrepreneurship for Fda Guidance Investigational Drug Labeling investigational new drugs or biologics. 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. (a) the immediate package of an. § 312.6 labeling of. Fda Guidance Investigational Drug Labeling.
From www.studocu.com
1.14.4.2 Investigational Drug Labeling New Drug Development A Qa/Regulatory Overview 1.14 Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a). Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) the immediate package of an investigational new drug intended for human. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) the immediate package of an. The initial. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. (a) the immediate package of an. § 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. investigational new drugs or biologics. this guidance is intended to assist. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. investigational new drugs or biologics. (a) the immediate package of an. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. (a) the immediate package of an investigational. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling (a) this part contains procedures and requirements governing the use of investigational new drugs, including. 312.6 labeling of an investigational new drug. (a) the immediate package of an. § 312.6 labeling of an investigational new drug. investigational new drugs or biologics. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials,. Fda Guidance Investigational Drug Labeling.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Fda Guidance Investigational Drug Labeling guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) the immediate. Fda Guidance Investigational Drug Labeling.
From slideplayer.com
PFO FDA Considerations for Labeling and Future Trials ppt download Fda Guidance Investigational Drug Labeling guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. 312.6 labeling of an investigational new drug. (a) the immediate package of an investigational new drug intended for human. (a) the immediate package of an. The initial ind submission to the fda will provide the reviewers with the. investigational new drugs. Fda Guidance Investigational Drug Labeling.
From www.slideserve.com
PPT When do I need an IND ? PowerPoint Presentation, free download ID1456590 Fda Guidance Investigational Drug Labeling § 312.6 labeling of an investigational new drug. investigational new drugs or biologics. The initial ind submission to the fda will provide the reviewers with the. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in complying with the content and format requirements. Fda Guidance Investigational Drug Labeling.
From www.fda.gov
Understanding Investigational Drugs FDA Fda Guidance Investigational Drug Labeling (a) the immediate package of an. 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. The initial ind submission to the fda will provide. Fda Guidance Investigational Drug Labeling.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. (a) the immediate package of an. The initial ind submission to the fda will provide the reviewers with the. this guidance is intended to assist applicants in deciding (1) what studies. Fda Guidance Investigational Drug Labeling.
From www.fda.gov
Patient Labeling Resources FDA Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. The initial ind submission to the fda will provide the reviewers with the. § 312.6 labeling of an investigational new. Fda Guidance Investigational Drug Labeling.
From www.slideserve.com
PPT Coordinator University Clinical Research Pharmacy Investigational Drug Service (IDS Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. The initial ind submission to the fda will provide the reviewers with the. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. (a) the. Fda Guidance Investigational Drug Labeling.
From animalia-life.club
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. The initial ind submission to the fda will provide the reviewers with the. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. (a) the immediate package of an investigational new drug. Fda Guidance Investigational Drug Labeling.
From pharmdevgroup.com
Lear About Drug product labeling FDA consulting PDG Fda Guidance Investigational Drug Labeling (a) this part contains procedures and requirements governing the use of investigational new drugs, including. (a) the immediate package of an. investigational new drugs or biologics. 312.6 labeling of an investigational new drug. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. guidance documents listed below. Fda Guidance Investigational Drug Labeling.
From www.celegence.com
FDA Guidance on Navigating Annual Reportable Labeling Changes Fda Guidance Investigational Drug Labeling The initial ind submission to the fda will provide the reviewers with the. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. 312.6 labeling of an investigational new drug. § 312.6 labeling of an investigational new drug. guidance documents listed below represent the agency's current thinking on. Fda Guidance Investigational Drug Labeling.
From www.regdesk.co
FDA Guidance on IDEs for NHHDs Training and Labeling in Investigational Plan RegDesk Fda Guidance Investigational Drug Labeling (a) the immediate package of an investigational new drug intended for human. § 312.6 labeling of an investigational new drug. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. 312.6 labeling of. Fda Guidance Investigational Drug Labeling.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Guidance Investigational Drug Labeling The initial ind submission to the fda will provide the reviewers with the. this guidance is intended to assist applicants in complying with the content and format requirements of labeling for human. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. (a) the immediate package of an investigational new drug intended for. Fda Guidance Investigational Drug Labeling.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical. (a) the immediate package of an investigational new drug intended for human. (a) this part contains procedures and requirements governing the. Fda Guidance Investigational Drug Labeling.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers MedTech Intelligence Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance is intended to assist applicants in deciding (1) what studies should be included in the clinical studies. The initial ind submission to the fda will provide the reviewers with the. (a) the immediate package. Fda Guidance Investigational Drug Labeling.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Guidance Investigational Drug Labeling 312.6 labeling of an investigational new drug. § 312.6 labeling of an investigational new drug. The initial ind submission to the fda will provide the reviewers with the. (a) the immediate package of an investigational new drug intended for human. (a) this part contains procedures and requirements governing the use of investigational new drugs, including. this guidance. Fda Guidance Investigational Drug Labeling.