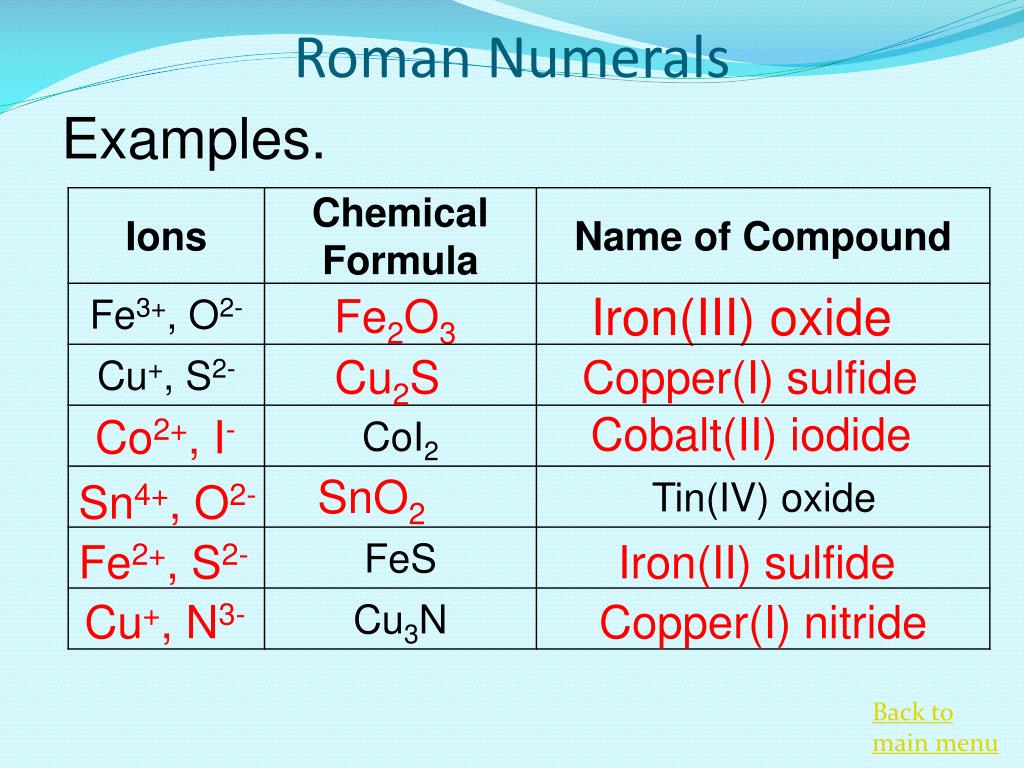
Why Do You Use Roman Numerals For Naming Compounds . This video explains how to name covalent and ionic compounds. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii).
from www.slideserve.com
roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. This video explains how to name covalent and ionic compounds.
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free
Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). This video explains how to name covalent and ionic compounds. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion.
From www.youtube.com
Naming Ionic Compounds with Roman Numerals YouTube Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element name followed by a roman numeral in parentheses if the. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation ID2090187 Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal cation forms more than. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
Naming Ionic Compounds with Roman Numerals! YouTube Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal cation forms more than. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Lecture 7 Ionic Compounds PowerPoint Presentation, free download Why Do You Use Roman Numerals For Naming Compounds This video explains how to name covalent and ionic compounds. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the transition metals below. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
Formula Writing for Ionic Compounds WITH Roman Numerals YouTube Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). This video explains how to name covalent and ionic compounds. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). This video explains how to name covalent and ionic compounds. the. Why Do You Use Roman Numerals For Naming Compounds.
From slideplayer.com
Chapter 3 Chemical Compounds. ppt download Why Do You Use Roman Numerals For Naming Compounds This video explains how to name covalent and ionic compounds. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the cation is the element name followed by a roman numeral in. Why Do You Use Roman Numerals For Naming Compounds.
From www.showme.com
Roman Numeral Ionic Compound Naming Science, Atoms ShowMe Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. This video explains how to name covalent and ionic compounds. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element. Why Do You Use Roman Numerals For Naming Compounds.
From www.reddit.com
How do I know when to use a roman numeral or prefix when naming in Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the transition metals below do not need a roman numeral in the names of their compounds. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. This video explains how to name covalent and ionic compounds. roman. Why Do You Use Roman Numerals For Naming Compounds.
From www.geeksforgeeks.org
Roman Numerals 1 to 1000 Roman Numbers Chart, Rules & Examples Why Do You Use Roman Numerals For Naming Compounds the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii). Why Do You Use Roman Numerals For Naming Compounds.
From slideplayer.com
Naming Compounds and Writing Formulas ppt download Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Guide to Naming Ionic Compounds PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. This video explains. Why Do You Use Roman Numerals For Naming Compounds.
From socratic.org
In a Stock system name such as iron (III) sulfate, what do the Roman Why Do You Use Roman Numerals For Naming Compounds the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). in the simpler, more modern approach, called the stock system, an ion’s positive charge. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Ionic Bonding and Nomenclature PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. This video explains how to name covalent and ionic compounds. the transition metals below do not need a roman numeral in the. Why Do You Use Roman Numerals For Naming Compounds.
From lessonlibmachinable.z22.web.core.windows.net
Roman Numerals To 1000 Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated. Why Do You Use Roman Numerals For Naming Compounds.
From romannumerals.site
Free Printable Roman Numerals Chart /Roman Number Chart Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). This video explains how to name covalent and ionic compounds. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the transition metals below. Why Do You Use Roman Numerals For Naming Compounds.
From templates.hilarious.edu.np
Roman Numerals Chart Printable Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the transition metals below do not need a roman numeral in the names of. Why Do You Use Roman Numerals For Naming Compounds.
From slideplayer.com
Naming Compounds and Writing Formulas ppt download Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds. Why Do You Use Roman Numerals For Naming Compounds.
From mungfali.com
Roman Numerals Explained Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. This video explains how to name covalent and ionic compounds. in the simpler, more modern approach,. Why Do You Use Roman Numerals For Naming Compounds.
From mungfali.com
Roman Numerals Explained Why Do You Use Roman Numerals For Naming Compounds This video explains how to name covalent and ionic compounds. roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). in the simpler, more modern approach,. Why Do You Use Roman Numerals For Naming Compounds.
From www.compoundworksheets.com
Naming Ionic Compounds With Roman Numerals Worksheet Worksheet Resume Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. This video explains how to name covalent and ionic compounds. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the transition metals below do not need a roman numeral in the. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
compound formulas name has roman numeral YouTube Why Do You Use Roman Numerals For Naming Compounds This video explains how to name covalent and ionic compounds. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). in the simpler, more. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Naming Compounds PowerPoint Presentation, free download ID2170250 Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated. Why Do You Use Roman Numerals For Naming Compounds.
From www.compoundworksheets.com
How To Name Ionic Compounds With Roman Numerals Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. This video explains how to name covalent and ionic compounds. roman numerals are used in naming ionic compounds when the. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
Naming Compounds using Roman Numerals YouTube Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. This video explains how to name covalent and ionic compounds. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using. Why Do You Use Roman Numerals For Naming Compounds.
From www.numerade.com
SOLVEDWhy must roman numerals be used when naming certain ionic compounds? Why Do You Use Roman Numerals For Naming Compounds the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. This video explains how to name covalent and ionic compounds. in the simpler, more. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
Naming Ionic Compound with and without roman numeral YouTube Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. This video explains how to name covalent and ionic compounds. in the simpler, more modern approach, called the. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Naming ionic compounds part II PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the cation is the element name followed by a roman numeral in parentheses if the element has multiple charges. the transition metals below do not need a roman numeral in the names of their compounds because they only form one. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Chemical Equations & Reactions PowerPoint Presentation, free Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which. Why Do You Use Roman Numerals For Naming Compounds.
From www.youtube.com
Naming Roman Numeral Ionic Compounds YouTube Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. This video explains how to name covalent and ionic compounds. the charge on a transition metal cation can. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideshare.net
Unit 12 Chemical Naming and Formulas Why Do You Use Roman Numerals For Naming Compounds in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). roman numerals are used in naming ionic compounds when the metal cation forms more than. Why Do You Use Roman Numerals For Naming Compounds.
From www.slideserve.com
PPT Writing Formulas & Naming Compounds PowerPoint Presentation ID Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. in the simpler, more modern approach, called the stock system, an ion’s positive charge is indicated by a. the cation is the element name followed by a roman numeral in parentheses if the element has. Why Do You Use Roman Numerals For Naming Compounds.
From dxonibtfh.blob.core.windows.net
What Do Roman Numerals Represent In Chemistry at Holland blog Why Do You Use Roman Numerals For Naming Compounds the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. This video explains how to name covalent and ionic compounds. the charge on a transition metal cation can also be indicated using roman numerals in parentheses, which looks like fe (ii) or fe (iii). the. Why Do You Use Roman Numerals For Naming Compounds.
From romansnumerals.com
Roman Numerals 1 To 5000 Roman Numerals Pro Why Do You Use Roman Numerals For Naming Compounds roman numerals are used in naming ionic compounds when the metal cation forms more than one ion. the transition metals below do not need a roman numeral in the names of their compounds because they only form one ion. This video explains how to name covalent and ionic compounds. the cation is the element name followed by. Why Do You Use Roman Numerals For Naming Compounds.