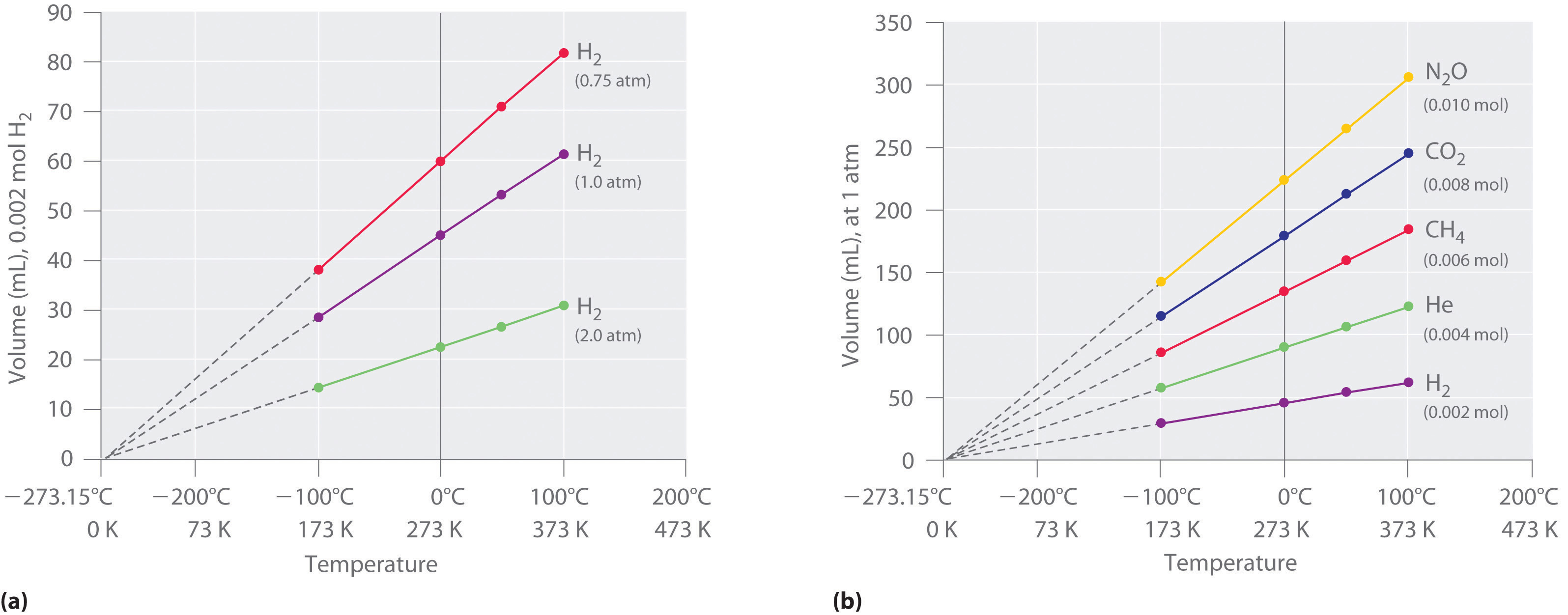
Constant Pressure And Absolute Temperature . The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). For an ideal gas, this means the volume of a gas is proportional. An isobaric process is a thermodynamic process in which pressure stays constant: \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. An ideal gas can be characterized by three state variables: Absolute pressure (p), volume (v), and absolute temperature (t). The volume of a given gas sample.
from chem.libretexts.org
An isobaric process is a thermodynamic process in which pressure stays constant: \ (\mathrm {δp = 0}\). An ideal gas can be characterized by three state variables: The volume of a given gas sample. For an ideal gas, this means the volume of a gas is proportional. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Absolute pressure (p), volume (v), and absolute temperature (t). The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law).
6.3 Relationships among Pressure, Temperature, Volume, and Amount Chemistry LibreTexts
Constant Pressure And Absolute Temperature Absolute pressure (p), volume (v), and absolute temperature (t). Absolute pressure (p), volume (v), and absolute temperature (t). An ideal gas can be characterized by three state variables: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The volume of a given gas sample. An isobaric process is a thermodynamic process in which pressure stays constant: For an ideal gas, this means the volume of a gas is proportional. \ (\mathrm {δp = 0}\). The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law).
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At Constant Temperature Cartoon Constant Pressure And Absolute Temperature Absolute pressure (p), volume (v), and absolute temperature (t). For an ideal gas, this means the volume of a gas is proportional. The volume of a given gas sample. An isobaric process is a thermodynamic process in which pressure stays constant: An ideal gas can be characterized by three state variables: At constant pressure, the volume of a fixed amount. Constant Pressure And Absolute Temperature.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial YouTube Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. An ideal gas can be characterized by three state variables: The volume of a given gas sample. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume. Constant Pressure And Absolute Temperature.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Chemistry LibreTexts Constant Pressure And Absolute Temperature An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute temperature (t). An ideal gas can be characterized by three state variables: The volume of a given gas sample. \ (\mathrm {δp = 0}\). For an ideal gas, this means the volume of a gas is proportional. At constant pressure, the. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation, free download ID1793338 Constant Pressure And Absolute Temperature The volume of a given gas sample. \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is a thermodynamic process in which pressure stays constant: The pressure of a given amount of gas. Constant Pressure And Absolute Temperature.
From slideplayer.com
Gas Law Unit. ppt download Constant Pressure And Absolute Temperature The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An isobaric process is a thermodynamic process in which pressure stays constant: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. For an ideal gas, this. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Chapter 12 The Laws of Thermodynamics PowerPoint Presentation, free download ID1117747 Constant Pressure And Absolute Temperature The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). The volume of a given gas sample. An ideal gas can be characterized by three state variables: An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute. Constant Pressure And Absolute Temperature.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Chemistry LibreTexts Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. An isobaric process is a thermodynamic process in which pressure stays constant: The volume of a given gas sample. \ (\mathrm {δp = 0}\). Absolute pressure (p), volume (v), and absolute temperature (t). At constant pressure, the volume of a fixed amount of gas is directly proportional. Constant Pressure And Absolute Temperature.
From www.youtube.com
Constant Pressure Heating of a gas YouTube Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. An isobaric process is a thermodynamic process in which pressure stays constant: An ideal gas can be characterized by three state variables: \ (\mathrm {δp = 0}\). The volume of a given gas sample. At constant pressure, the volume of a fixed amount of gas is directly. Constant Pressure And Absolute Temperature.
From socratic.org
Which graph shows the relationship between the temperature and volume of a gas according to Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. An isobaric process is a thermodynamic process in which pressure stays constant: \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Absolute pressure (p), volume (v), and absolute temperature (t). The pressure. Constant Pressure And Absolute Temperature.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Chemistry Constant Pressure And Absolute Temperature An ideal gas can be characterized by three state variables: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Absolute pressure (p), volume (v), and absolute temperature (t). For an ideal gas, this means the volume of a gas is proportional. The volume of a given gas sample. \ (\mathrm. Constant Pressure And Absolute Temperature.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas. YouTube Constant Pressure And Absolute Temperature An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute temperature (t). The volume of a given gas sample. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). For an ideal gas, this means the volume. Constant Pressure And Absolute Temperature.
From www.grc.nasa.gov
Heat Transfer Constant Pressure And Absolute Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. \ (\mathrm {δp = 0}\). Absolute pressure (p), volume (v), and absolute temperature (t). The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An ideal gas. Constant Pressure And Absolute Temperature.
From courses.lumenlearning.com
Phase Changes Physics Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. \ (\mathrm {δp = 0}\). The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An isobaric process is a thermodynamic process in which pressure stays constant: An ideal gas can be characterized. Constant Pressure And Absolute Temperature.
From www.numerade.com
SOLVED The osmotic pressure of a solution is calculated using the formula Π=MRT where Π is the Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). An ideal gas can be characterized by three state variables: An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute temperature (t). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. For an ideal gas, this. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation ID6039549 Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An ideal gas can be characterized by three state variables: An isobaric process is a thermodynamic process in which pressure stays constant: At. Constant Pressure And Absolute Temperature.
From www.numerade.com
SOLVED Which of the following graphs shows the correct relationship between the pressure and Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). The volume of a given gas sample. An ideal gas can be characterized by three state variables: The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An isobaric process is a thermodynamic process in which pressure stays constant: At constant. Constant Pressure And Absolute Temperature.
From schematichonorable.z21.web.core.windows.net
Tv Diagram Constant Pressure Constant Pressure And Absolute Temperature The volume of a given gas sample. For an ideal gas, this means the volume of a gas is proportional. An ideal gas can be characterized by three state variables: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The pressure of a given amount of gas is directly proportional. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Presentation ID3207761 Constant Pressure And Absolute Temperature The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). The volume of a given gas sample. An isobaric process is a thermodynamic process in which pressure stays constant: An ideal gas can be characterized by three state variables: Absolute pressure (p), volume (v), and absolute. Constant Pressure And Absolute Temperature.
From www.toppr.com
The heat of reaction C(s) + 12O2(g) CO(g) at 17^oC and at constant volume is 29.29 kcal Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The volume of a given gas sample. Absolute pressure (p), volume (v), and absolute temperature (t). An ideal gas can be characterized by three state variables: An isobaric process is a thermodynamic process in which pressure. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Heating at constant pressure PowerPoint Presentation, free download ID6782399 Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). An isobaric process is a thermodynamic process in which pressure stays constant: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law).. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Zeroth Law of Thermodynamics PowerPoint Presentation, free download ID4959841 Constant Pressure And Absolute Temperature Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is a thermodynamic process in which pressure stays constant: \ (\mathrm {δp = 0}\). An ideal gas can be characterized by three state variables: For an ideal gas, this means the volume of a gas is proportional. At constant pressure, the volume of a fixed amount of gas. Constant Pressure And Absolute Temperature.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Constant Pressure And Absolute Temperature An isobaric process is a thermodynamic process in which pressure stays constant: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. \ (\mathrm {δp = 0}\). The volume of a given gas sample. Absolute pressure (p), volume (v), and absolute temperature (t). For an ideal gas, this means the volume. Constant Pressure And Absolute Temperature.
From www.grc.nasa.gov
Equation of State Constant Pressure And Absolute Temperature The volume of a given gas sample. \ (\mathrm {δp = 0}\). An ideal gas can be characterized by three state variables: An isobaric process is a thermodynamic process in which pressure stays constant: For an ideal gas, this means the volume of a gas is proportional. The pressure of a given amount of gas is directly proportional to its. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Temperature and Ideal Gas PowerPoint Presentation, free download ID1283632 Constant Pressure And Absolute Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. For an ideal gas, this means the volume of a gas is proportional. \ (\mathrm {δp = 0}\). An isobaric process is a thermodynamic process in which pressure stays constant: The pressure of a given amount of gas is directly proportional. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download ID6717792 Constant Pressure And Absolute Temperature An ideal gas can be characterized by three state variables: \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. For an ideal gas, this means the volume of a gas is proportional. Absolute pressure (p), volume (v), and absolute temperature (t). The volume of a. Constant Pressure And Absolute Temperature.
From www.youtube.com
Change in ENTHALPY Using Specific Heat at Constant Pressure in 3 Minutes! YouTube Constant Pressure And Absolute Temperature The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute temperature (t). The volume of a given gas sample. \ (\mathrm {δp = 0}\). At constant pressure,. Constant Pressure And Absolute Temperature.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector Illustration of formula Constant Pressure And Absolute Temperature The volume of a given gas sample. An ideal gas can be characterized by three state variables: The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). \ (\mathrm {δp = 0}\). An isobaric process is a thermodynamic process in which pressure stays constant: For an. Constant Pressure And Absolute Temperature.
From www.pinterest.co.uk
A mathematical derivation of the equations relating the pressure, temperature, and volume during Constant Pressure And Absolute Temperature \ (\mathrm {δp = 0}\). An ideal gas can be characterized by three state variables: The volume of a given gas sample. An isobaric process is a thermodynamic process in which pressure stays constant: Absolute pressure (p), volume (v), and absolute temperature (t). The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Unit 5 Gases More Gas Laws Charles’s Law and Boyle’s Law PowerPoint Presentation ID Constant Pressure And Absolute Temperature At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. The volume of a given gas sample. The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). An isobaric process is a thermodynamic process in which pressure. Constant Pressure And Absolute Temperature.
From www.grc.nasa.gov
Specific Heats Constant Pressure And Absolute Temperature An isobaric process is a thermodynamic process in which pressure stays constant: An ideal gas can be characterized by three state variables: \ (\mathrm {δp = 0}\). For an ideal gas, this means the volume of a gas is proportional. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. Absolute. Constant Pressure And Absolute Temperature.
From www.researchgate.net
A Pressure/Temperature equilibrium phase diagram of a one component... Download Scientific Diagram Constant Pressure And Absolute Temperature Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is a thermodynamic process in which pressure stays constant: At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. An ideal gas can be characterized by three state variables: \ (\mathrm {δp = 0}\). The volume of a given. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Constant Pressure And Absolute Temperature The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (amontons’s law). Absolute pressure (p), volume (v), and absolute temperature (t). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. An ideal gas can be characterized by three. Constant Pressure And Absolute Temperature.
From slideplayer.com
Ch. 8 phases gases,liquids,and solids ppt download Constant Pressure And Absolute Temperature Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is a thermodynamic process in which pressure stays constant: The volume of a given gas sample. An ideal gas can be characterized by three state variables: For an ideal gas, this means the volume of a gas is proportional. The pressure of a given amount of gas is. Constant Pressure And Absolute Temperature.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6307179 Constant Pressure And Absolute Temperature An ideal gas can be characterized by three state variables: \ (\mathrm {δp = 0}\). At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. For an ideal gas, this means the volume of a gas is proportional. Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is. Constant Pressure And Absolute Temperature.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Constant Pressure And Absolute Temperature For an ideal gas, this means the volume of a gas is proportional. At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature (in. An ideal gas can be characterized by three state variables: Absolute pressure (p), volume (v), and absolute temperature (t). An isobaric process is a thermodynamic process in which. Constant Pressure And Absolute Temperature.