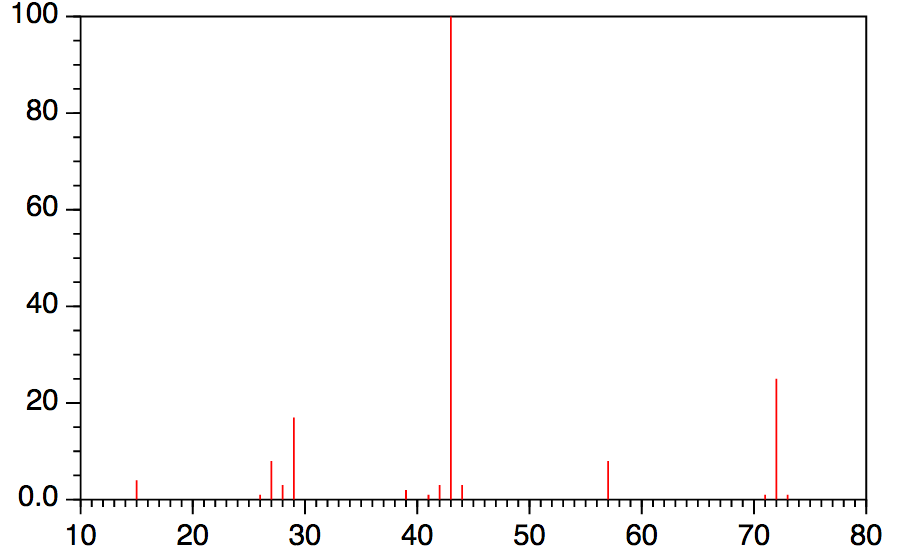
Mass Spectrometry Organic Chemistry . Which is the base peak? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which peak represents the m +?
from scilearn.sydney.edu.au
Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? Which peak represents the m +? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which is the base peak?
Spectroscopy in Organic Chemistry Mass Spectrometry
Mass Spectrometry Organic Chemistry Which is the base peak? Which is the base peak? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Which peak represents the m +? It is designed for students of. Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry M Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Why is there a peak at 122? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. It is designed for students of. Learn how mass spectrometry is used to identify unknown. Mass Spectrometry Organic Chemistry.
From app.jove.com
Mass Spectrometry of Amines Concept Organic Chemistry JoVe Mass Spectrometry Organic Chemistry Which is the base peak? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. At its simplest, mass spectrometry (ms) is a technique for measuring the mass,. Mass Spectrometry Organic Chemistry.
From www.youtube.com
Mass spectroscopy "part 1" Organic Chemistry YouTube Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which is the base peak? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Which peak represents the m +? Chad introduces mass spectrometry breaking down a variety of terms including. Mass Spectrometry Organic Chemistry.
From www.compoundchem.com
Mass Spectrometry and Interpreting Mass Spectra Compound Interest Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Which is the base peak? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent.. Mass Spectrometry Organic Chemistry.
From organicchemistoncall.com
Most Commonly Used IR Spectroscopy Values In Organic Chemistry The Mass Spectrometry Organic Chemistry Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Which is the base peak? Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. It is designed for students of.. Mass Spectrometry Organic Chemistry.
From www.youtube.com
Organic Chemistry Mass Spectrometry Part I YouTube Mass Spectrometry Organic Chemistry It is designed for students of. Why is there a peak at 122? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which is the base peak? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would be. Mass Spectrometry Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Multiplet Mass Spectrometry Organic Chemistry It is designed for students of. Why is there a peak at 122? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Which is the base. Mass Spectrometry Organic Chemistry.
From www.pinterest.com
Khan Academy Mass spectrometry, Spectrometers, Physics and mathematics Mass Spectrometry Organic Chemistry Which peak represents the m +? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. It is designed for students of. Learn how mass. Mass Spectrometry Organic Chemistry.
From chem.libretexts.org
Mass spectrometry 1 Chemistry LibreTexts Mass Spectrometry Organic Chemistry It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. At its simplest, mass spectrometry. Mass Spectrometry Organic Chemistry.
From scilearn.sydney.edu.au
Spectroscopy in Organic Chemistry Mass Spectrometry Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. It is designed for students of. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Why is there a peak at 122? Chad introduces mass spectrometry breaking down a variety. Mass Spectrometry Organic Chemistry.
From www.wizeprep.com
Mass Spectrometry Wize University Organic Chemistry Textbook Wizeprep Mass Spectrometry Organic Chemistry Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. It is designed for students of. Which peak represents the m. Mass Spectrometry Organic Chemistry.
From chem.libretexts.org
B2. Sequence Determination Using Mass Spectrometry Chemistry LibreTexts Mass Spectrometry Organic Chemistry Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. It is designed for students of. Why is there a peak at 122? Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Chad introduces mass spectrometry. Mass Spectrometry Organic Chemistry.
From www.pinterest.com
Pin on Chemie Mass Spectrometry Organic Chemistry Which is the base peak? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Which peak represents the m +? Mass spectrometry would be useful even if molecular weight. Mass Spectrometry Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry NMR spectrum Mass Spectrometry Organic Chemistry Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Why is there a peak at 122? Which peak represents the m +? Which is the base peak? It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would. Mass Spectrometry Organic Chemistry.
From www.researchgate.net
Spectrums of mass spectrometry for five compounds identification after Mass Spectrometry Organic Chemistry Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Why is there a peak at 122? It is designed for students of. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to. Mass Spectrometry Organic Chemistry.
From mungfali.com
Mass Spectrometry Spectrum Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Which peak represents the m +? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the. Mass Spectrometry Organic Chemistry.
From www.youtube.com
Mass Spectrometry in Organic Chemistry // HSC Chemistry YouTube Mass Spectrometry Organic Chemistry Why is there a peak at 122? Which is the base peak? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would be. Mass Spectrometry Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Spectrum Mass Spectrometry Organic Chemistry Which is the base peak? It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which peak represents the m +? Learn how mass spectrometry is. Mass Spectrometry Organic Chemistry.
From www.nature.com
Global chemical analysis of biology by mass spectrometry Nature Mass Spectrometry Organic Chemistry It is designed for students of. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Which is the base peak? Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Learn how mass spectrometers measure the. Mass Spectrometry Organic Chemistry.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Mass Spectrometry Organic Chemistry Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Which is the base peak? Why is there a peak at 122? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the. Mass Spectrometry Organic Chemistry.
From www.chemistryviews.org
Mass Spectrometry of Volatile Organic Compounds ChemistryViews Mass Spectrometry Organic Chemistry Why is there a peak at 122? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Which peak represents the m +? Which is the base peak? Mass spectrometry would be. Mass Spectrometry Organic Chemistry.
From in.pinterest.com
Mass Spectrometry and IR Spectroscopy Organic chemistry, Chemistry Mass Spectrometry Organic Chemistry Which is the base peak? Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which peak represents the m +? Learn how mass spectrometry is used to identify unknown compounds, interpret. Mass Spectrometry Organic Chemistry.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectrometry Organic Chemistry Why is there a peak at 122? It is designed for students of. Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. At its simplest, mass spectrometry. Mass Spectrometry Organic Chemistry.
From mavink.com
Mass Spectrometry Cheat Sheet Mass Spectrometry Organic Chemistry Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Which is the base peak? At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. It is designed for students of. Learn. Mass Spectrometry Organic Chemistry.
From www.studocu.com
1 (10) Mass Spectrometry and IR Spectroscopy Section 11 of Organic Mass Spectrometry Organic Chemistry Which is the base peak? It is designed for students of. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. At its simplest, mass spectrometry (ms). Mass Spectrometry Organic Chemistry.
From www.savemyexams.co.uk
Mass spectrometry (3.6.2) AQA A Level Chemistry Revision Notes 2017 Mass Spectrometry Organic Chemistry Why is there a peak at 122? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Which peak represents the m +? Learn how mass spectrometers measure. Mass Spectrometry Organic Chemistry.
From www.docbrown.info
mass spectrum of propyl methanoate C4H8O2 HCOOCH2CH2CH3 fragmentation Mass Spectrometry Organic Chemistry Which is the base peak? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Chad introduces mass spectrometry breaking down a variety of terms including the base. Mass Spectrometry Organic Chemistry.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which peak represents the m +? Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would be useful even if molecular weight and formula were the only information that. Mass Spectrometry Organic Chemistry.
From www.researchgate.net
Mass spectra of intermediates with m/z 28, 42, 56, 70, 84, 98, and 112 Mass Spectrometry Organic Chemistry Why is there a peak at 122? Which peak represents the m +? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. It is designed for students of. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions. Mass Spectrometry Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Gas chromatographymass Mass Spectrometry Organic Chemistry Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Why is there a peak at 122? It is designed for students of. At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore. Mass Spectrometry Organic Chemistry.
From www.artofit.org
Organic chemistry notes mass spectrometry and ir spectroscopy Artofit Mass Spectrometry Organic Chemistry Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Chad introduces mass spectrometry breaking down a variety of terms including the base peak, the parent. Which peak. Mass Spectrometry Organic Chemistry.
From www.organicchemistrytutor.com
Spectroscopy Cheat Sheets — Organic Chemistry Tutor Mass Spectrometry Organic Chemistry Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. It is designed for students of. Which peak represents the m +? Chad introduces mass spectrometry breaking. Mass Spectrometry Organic Chemistry.
From chem.libretexts.org
4.1 Mass Spectrometry Chemistry LibreTexts Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which is the base peak? Learn how mass spectrometry is used to identify unknown compounds, interpret mass spectra, and deduce molecular formula. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and.. Mass Spectrometry Organic Chemistry.
From www.tes.com
AS Chemistry Mass Spectrometry in Organic Chemistry Teaching Resources Mass Spectrometry Organic Chemistry Which is the base peak? Why is there a peak at 122? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Learn how mass spectrometers measure the characteristics of individual molecules by converting them to ions and. Which peak represents the m +?. Mass Spectrometry Organic Chemistry.
From pinterest.com
Mass spectrometry simple diagram ChemistrySpectroscopy and Mass Spectrometry Organic Chemistry At its simplest, mass spectrometry (ms) is a technique for measuring the mass, and therefore the molecular weight (mw), of a molecule. Which is the base peak? Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. Which peak represents the m +? Learn. Mass Spectrometry Organic Chemistry.