Is Ammonium Carbonate An Acid Or Base . Acidic/basic nature of a salt solution of a. a base is a substance that can react with an acid and neutralise it. ask your own question! — carbonate is a moderately strong base. relative strength of acids & bases. acids react with metal carbonates and hydrogencarbonates in the same way. to tell if (nh4)2co3 (ammonium carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. Alkali metals can be mined in the form: These reactions produce salt, water and carbon. if it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. It has a role as a raising agent and a food. — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. carbonate ions from the carbonate react with hydrogen ions from the acid. Acids react with metals, bases and carbonates to produce.
from courses.lumenlearning.com
ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. ask your own question! relative strength of acids & bases. indicators are used to determine whether a solution is acidic or alkaline. carbonate ions from the carbonate react with hydrogen ions from the acid. a base is a substance that can react with an acid and neutralise it. Acidic/basic nature of a salt solution of a. Na 2 co 3, sodium carbonate. That means the base and the acid cancel each other out. — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in.
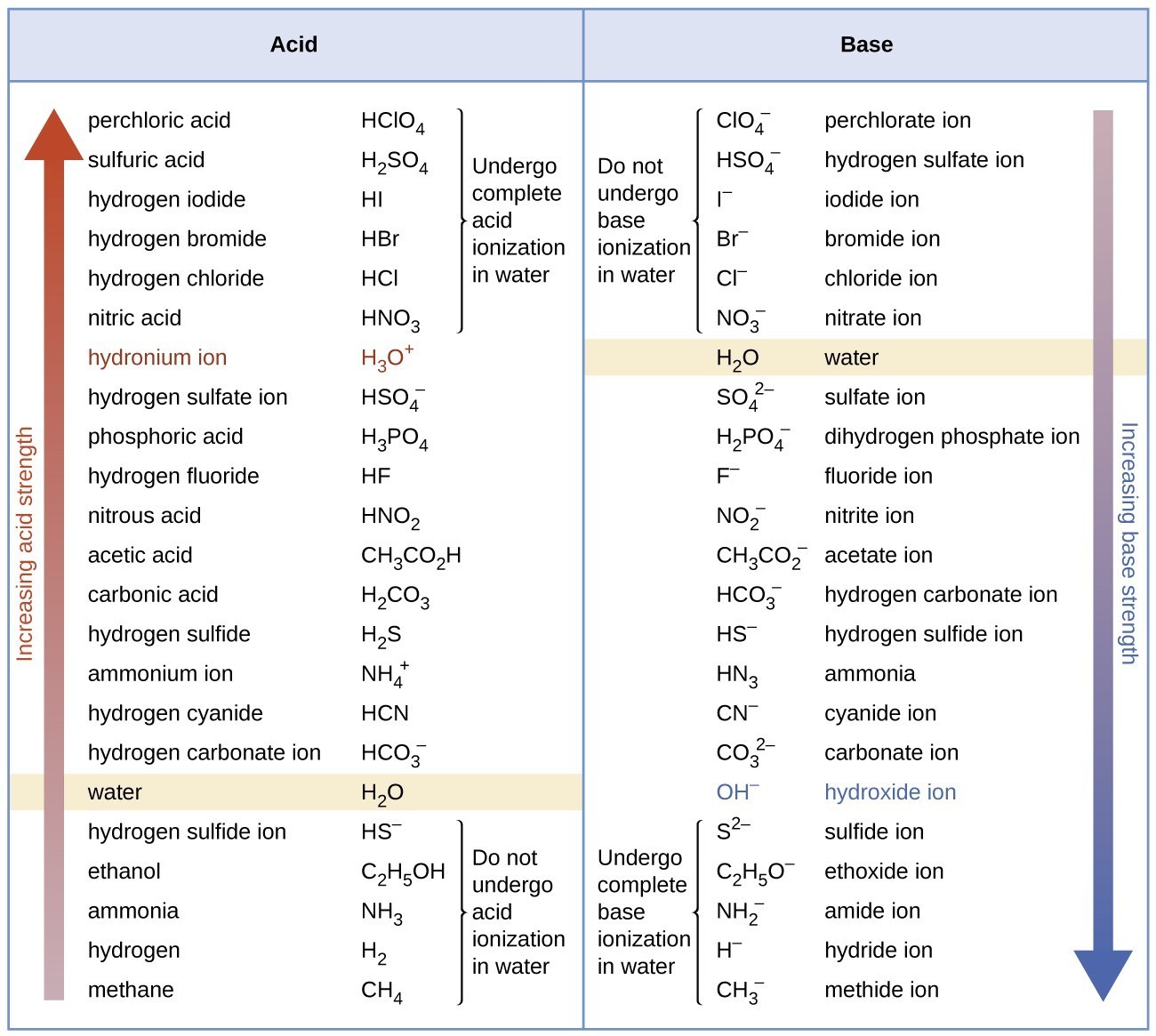
Relative Strengths of Acids and Bases Chemistry
Is Ammonium Carbonate An Acid Or Base indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce. — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. Alkali metals can be mined in the form: — carbonate is a moderately strong base. These reactions produce salt, water and carbon. ammonium carbonate is a white solid. Use this acids and bases chart to find the relative strength of the most common acids and bases. It reacts with acids to make an ammonium salt and carbon. It has a role as a raising agent and a food. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. Na 2 co 3, sodium carbonate. — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. as we all know that all the chemical compounds can be put in three categories namely acids, bases, and salts; a base is a substance that can react with an acid and neutralise it.
From www.informationhospitaliere.com
Carbonate d'ammonium utilisations et dangers de que savoir de l Is Ammonium Carbonate An Acid Or Base if it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. — carbonate is a moderately strong base. ask your own question! a base is a substance that can react with an acid and neutralise it. relative strength of acids. Is Ammonium Carbonate An Acid Or Base.
From sujatanutripharma.com
Ammonium Carbonate Sujata Nutri Pharma Is Ammonium Carbonate An Acid Or Base if it is covalent, which is typically between 2 or more nonmetals, we need to ask, is it a simple molecule, or is it an acid. ammonium carbonate is a white solid. Use this acids and bases chart to find the relative strength of the most common acids and bases. That means the base and the acid cancel. Is Ammonium Carbonate An Acid Or Base.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Is Ammonium Carbonate An Acid Or Base Aqueous solution of ammonium carbonate is basic. Acidic/basic nature of a salt solution of a. ammonium carbonate is a white solid. a base is a substance that can react with an acid and neutralise it. It has a role as a raising agent and a food. carbonate ions from the carbonate react with hydrogen ions from the. Is Ammonium Carbonate An Acid Or Base.
From www.indiamart.com
Ammonium Carbonate Powder, Technical Grade at Rs 2000/kg in Agra ID Is Ammonium Carbonate An Acid Or Base — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. relative strength of acids & bases. That means the base and the acid cancel each other out. a base is a substance that can react with an acid and neutralise it. Ammonium carbonate is a base. carbonate. Is Ammonium Carbonate An Acid Or Base.
From sciencing.com
Ammonium Carbonate Uses Sciencing Is Ammonium Carbonate An Acid Or Base — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. Alkali metals can be mined in the form: — carbonate is a moderately strong base. to tell if (nh4)2co3 (ammonium carbonate) is ionic or covalent (also called molecular) we look at the periodic. Is Ammonium Carbonate An Acid Or Base.
From www.youtube.com
Preparation & Properties of Ammonium carbonate YouTube Is Ammonium Carbonate An Acid Or Base reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. a base is a substance that can react with an acid and neutralise it. — carbonate is a moderately strong base. indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce.. Is Ammonium Carbonate An Acid Or Base.
From www.dreamstime.com
Ammonium Carbonate in Bottle, Chemical in the Laboratory and Industry Is Ammonium Carbonate An Acid Or Base Aqueous solution of ammonium carbonate is basic. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. Na 2 co 3, sodium carbonate. ammonium carbonate is a white solid. Acidic/basic nature of a salt solution of a. Alkali metals can be mined in the form: These reactions produce salt, water and carbon. relative strength of acids. Is Ammonium Carbonate An Acid Or Base.
From www.scribd.com
Ammonium Carbonate PDF Ammonium Salt (Chemistry) Is Ammonium Carbonate An Acid Or Base — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. Na 2 co 3, sodium carbonate. reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. That means the base and the acid cancel each other out. Use this acids. Is Ammonium Carbonate An Acid Or Base.
From testbook.com
Ammonium Carbonate Formula Concept, Structure, Properties & Uses. Is Ammonium Carbonate An Acid Or Base Na 2 co 3, sodium carbonate. That means the base and the acid cancel each other out. It has a role as a raising agent and a food. indicators are used to determine whether a solution is acidic or alkaline. carbonate ions from the carbonate react with hydrogen ions from the acid. Ammonium carbonate is a base. . Is Ammonium Carbonate An Acid Or Base.
From www.vrogue.co
Pka Of The Functional Groups Values To Know Pka Chart vrogue.co Is Ammonium Carbonate An Acid Or Base These reactions produce salt, water and carbon. ammonium carbonate is a white solid. relative strength of acids & bases. as we all know that all the chemical compounds can be put in three categories namely acids, bases, and salts; — carbonate is a moderately strong base. It reacts with acids to make an ammonium salt and. Is Ammonium Carbonate An Acid Or Base.
From www.funcmater.com
wholesale Ammonium carbonate Powder FUNCMATER Is Ammonium Carbonate An Acid Or Base Use this acids and bases chart to find the relative strength of the most common acids and bases. indicators are used to determine whether a solution is acidic or alkaline. Na 2 co 3, sodium carbonate. It has a role as a raising agent and a food. ammonium carbonate is a white solid. Alkali metals can be mined. Is Ammonium Carbonate An Acid Or Base.
From www.essecouk.com
AMMONIUM CARBONATE LUMP Esseco UK Is Ammonium Carbonate An Acid Or Base ammonium carbonate is a white solid. reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. — since acids and bases can be weak or strong there are four types of salts that can result, and these will. Is Ammonium Carbonate An Acid Or Base.
From www.ftfscientific.com
Ammonium Carbonate FTF Scientific Is Ammonium Carbonate An Acid Or Base — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. a base is a substance that can react with an acid and neutralise it. Ammonium carbonate is a base. indicators are used to determine whether a solution is acidic or alkaline. These reactions produce salt, water and. Is Ammonium Carbonate An Acid Or Base.
From www.numerade.com
SOLVED a solution of ammonium carbonate is allowed to react with an Is Ammonium Carbonate An Acid Or Base — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. Alkali metals can be mined in the form: These reactions produce salt, water and carbon. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. That means the base and the acid cancel each other out. It has. Is Ammonium Carbonate An Acid Or Base.
From www.fishersci.ca
Ammonium carbonate, ACS reagent, Thermo Scientific Chemicals Fisher Is Ammonium Carbonate An Acid Or Base reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. — carbonate is a moderately strong base. Acidic/basic nature of a salt solution of a. relative strength of acids & bases. Acids react with metals, bases and carbonates to produce. — since acids and bases can be weak or strong there are. Is Ammonium Carbonate An Acid Or Base.
From scienceforyou.netlify.app
Sodium carbonate acid or base scienceforyou Is Ammonium Carbonate An Acid Or Base relative strength of acids & bases. These reactions produce salt, water and carbon. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. Na 2 co 3, sodium carbonate. acids react with metal carbonates and hydrogencarbonates in the same way. reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. Aqueous. Is Ammonium Carbonate An Acid Or Base.
From shineshower30.gitlab.io
Fine Beautiful Ammonium Carbonate Balanced Equation Staar Geometry Is Ammonium Carbonate An Acid Or Base ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. Use this acids and bases chart to find the relative strength of the most common acids and bases. It reacts with acids to make an ammonium salt and carbon. relative strength of acids & bases. — ammonium carbonate is a compound with the. Is Ammonium Carbonate An Acid Or Base.
From www.pc-chem.com
Ammonium carbonate Is Ammonium Carbonate An Acid Or Base ask your own question! — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. It reacts with acids to make an ammonium salt and. Is Ammonium Carbonate An Acid Or Base.
From shop.kremerpigments.com
Ammonium Carbonate Substances Chemicals Solvents Is Ammonium Carbonate An Acid Or Base Aqueous solution of ammonium carbonate is basic. — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. — carbonate is a moderately strong base. These reactions produce salt, water and carbon. — however, as we have already discussed, the ammonium ion acts as. Is Ammonium Carbonate An Acid Or Base.
From apcpure.com
Ammonium Carbonate 30+ ACS APC Pure Is Ammonium Carbonate An Acid Or Base It reacts with acids to make an ammonium salt and carbon. ammonium carbonate is a white solid. relative strength of acids & bases. Use this acids and bases chart to find the relative strength of the most common acids and bases. a base is a substance that can react with an acid and neutralise it. —. Is Ammonium Carbonate An Acid Or Base.
From studylib.net
Reaction of Acids and Carbonates Is Ammonium Carbonate An Acid Or Base relative strength of acids & bases. — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in. reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. — however, as we have already discussed, the ammonium ion acts as. Is Ammonium Carbonate An Acid Or Base.
From www.teachoo.com
[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions] Is Ammonium Carbonate An Acid Or Base a base is a substance that can react with an acid and neutralise it. — ammonium carbonate is a compound with the chemical formula (nh4)2co3. Acids react with metals, bases and carbonates to produce. Acidic/basic nature of a salt solution of a. indicators are used to determine whether a solution is acidic or alkaline. — since. Is Ammonium Carbonate An Acid Or Base.
From www.huarongroup.com
Ammonium Acid Carbonate; Salvolatile Buy Ammonium Bicarbonate Is Ammonium Carbonate An Acid Or Base a base is a substance that can react with an acid and neutralise it. That means the base and the acid cancel each other out. — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. It reacts with acids to make an ammonium salt and carbon. acids. Is Ammonium Carbonate An Acid Or Base.
From www.sciencephoto.com
Ammonium carbonate Stock Image C043/6546 Science Photo Library Is Ammonium Carbonate An Acid Or Base a base is a substance that can react with an acid and neutralise it. to tell if (nh4)2co3 (ammonium carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see. That means the base and the acid cancel each other out. Aqueous solution of ammonium carbonate is basic. Alkali metals can be. Is Ammonium Carbonate An Acid Or Base.
From www.indiamart.com
Ammonium Carbonate Powder at Rs 50/kg Ammonium Carbonate in Surat Is Ammonium Carbonate An Acid Or Base Acids react with metals, bases and carbonates to produce. carbonate ions from the carbonate react with hydrogen ions from the acid. relative strength of acids & bases. a base is a substance that can react with an acid and neutralise it. — ammonium carbonate can react with acids and bases, and when it is reacted with. Is Ammonium Carbonate An Acid Or Base.
From stock.adobe.com
3D image of ammonium carbonate skeletal formula molecular chemical Is Ammonium Carbonate An Acid Or Base ask your own question! — carbonate is a moderately strong base. ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. These reactions produce salt, water and carbon. — since acids and bases can be weak or strong there are four types of salts that can result, and these will result in.. Is Ammonium Carbonate An Acid Or Base.
From www.youtube.com
NH42CO3 Plus H2O equation Ammonium carbonate YouTube Is Ammonium Carbonate An Acid Or Base ask your own question! — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. Ammonium carbonate is a base. Acidic/basic nature of a salt solution of a. reactions of acids with metals, carbonate and bicarbonate salts, and arrhenius bases are introduced. — ammonium carbonate can react with. Is Ammonium Carbonate An Acid Or Base.
From www.exportersindia.com
Ammonium Carbonate Hoxland Corporation, Haryana Is Ammonium Carbonate An Acid Or Base Aqueous solution of ammonium carbonate is basic. Use this acids and bases chart to find the relative strength of the most common acids and bases. acids react with metal carbonates and hydrogencarbonates in the same way. Acidic/basic nature of a salt solution of a. These reactions produce salt, water and carbon. reactions of acids with metals, carbonate and. Is Ammonium Carbonate An Acid Or Base.
From www.toppr.com
What is Ammonium Carbonate? Definition, Formulas, and Uses Is Ammonium Carbonate An Acid Or Base Alkali metals can be mined in the form: acids react with metal carbonates and hydrogencarbonates in the same way. — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. It has a role as a raising agent and a food. — carbonate is a moderately strong base.. Is Ammonium Carbonate An Acid Or Base.
From www.youtube.com
Is (NH4)2CO3 (Ammonium carbonate) Ionic or Covalent? YouTube Is Ammonium Carbonate An Acid Or Base to tell if (nh4)2co3 (ammonium carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see. indicators are used to determine whether a solution is acidic or alkaline. Ammonium carbonate is a base. Acids react with metals, bases and carbonates to produce. ammonium carbonate is an ammonium salt that is the. Is Ammonium Carbonate An Acid Or Base.
From www.carolina.com
Ammonium Carbonate, Reagent Grade, 500 g Carolina Biological Supply Is Ammonium Carbonate An Acid Or Base Aqueous solution of ammonium carbonate is basic. These reactions produce salt, water and carbon. Ammonium carbonate is a base. That means the base and the acid cancel each other out. — ammonium carbonate can react with acids and bases, and when it is reacted with an acid, we get ammonium. Na 2 co 3, sodium carbonate. acids react. Is Ammonium Carbonate An Acid Or Base.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Is Ammonium Carbonate An Acid Or Base These reactions produce salt, water and carbon. Na 2 co 3, sodium carbonate. — however, as we have already discussed, the ammonium ion acts as a weak acid in solution, while the. Use this acids and bases chart to find the relative strength of the most common acids and bases. Alkali metals can be mined in the form: . Is Ammonium Carbonate An Acid Or Base.
From www.youtube.com
Reactions between Metal Carbonates and Acids YouTube Is Ammonium Carbonate An Acid Or Base That means the base and the acid cancel each other out. ammonium carbonate is an ammonium salt that is the diammonium salt of carbonic acid. It reacts with acids to make an ammonium salt and carbon. — since acids and bases can be weak or strong there are four types of salts that can result, and these will. Is Ammonium Carbonate An Acid Or Base.
From www.goodscience.com.au
AcidBase Reactions Good Science Is Ammonium Carbonate An Acid Or Base — ammonium carbonate is a compound with the chemical formula (nh4)2co3. Acidic/basic nature of a salt solution of a. These reactions produce salt, water and carbon. acids react with metal carbonates and hydrogencarbonates in the same way. indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to. Is Ammonium Carbonate An Acid Or Base.
From first-labs.com
Ammonium carbonate Is Ammonium Carbonate An Acid Or Base carbonate ions from the carbonate react with hydrogen ions from the acid. These reactions produce salt, water and carbon. indicators are used to determine whether a solution is acidic or alkaline. to tell if (nh4)2co3 (ammonium carbonate) is ionic or covalent (also called molecular) we look at the periodic table that and see. That means the base. Is Ammonium Carbonate An Acid Or Base.