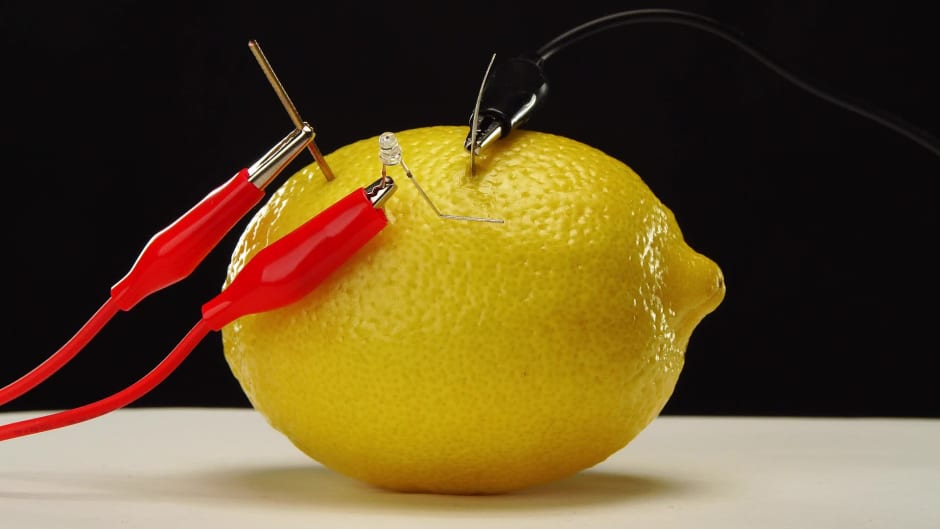
Electric Lemon Experiment . for projects like making a battery from a lemon, let students play around. But add electrodes, make a path for electrons to move, and you have all the basic elements. The citric acid of the lemon reacts with the zinc and loosens electrons. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. a lemon on its own is not a battery. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum.
from melscience.com
for projects like making a battery from a lemon, let students play around. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. a lemon on its own is not a battery. The citric acid of the lemon reacts with the zinc and loosens electrons. But add electrodes, make a path for electrons to move, and you have all the basic elements.
Lemon battery MEL Chemistry
Electric Lemon Experiment a lemon on its own is not a battery. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. for projects like making a battery from a lemon, let students play around. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. a lemon on its own is not a battery. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. But add electrodes, make a path for electrons to move, and you have all the basic elements.
From www.pinterest.fr
Organic electricity...from lemons? Electric Lemon science experiment Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. for projects like making a battery from a lemon, let students play around. the battery you. Electric Lemon Experiment.
From effectivebits.blogspot.com
Joel Avrunin's Effective Bits of Knowledge Batteries from Lemons A Electric Lemon Experiment the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. the battery you just. Electric Lemon Experiment.
From gosciencegirls.com
Lemon Light Experiment (How to Make a Lemon Battery) Go Science Girls Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. a lemon on its own is not a battery. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum.. Electric Lemon Experiment.
From wirefixcitemperance.z21.web.core.windows.net
Lemon Battery Circuit Diagram Electric Lemon Experiment the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. But add electrodes, make a path for electrons to move, and you have all the basic elements. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon.. Electric Lemon Experiment.
From americanwarmoms.org
How To Make A Light Bulb Work Using Lemon Electric Lemon Experiment The citric acid of the lemon reacts with the zinc and loosens electrons. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. the battery you made. Electric Lemon Experiment.
From www.rinconeducativo.org
Limones eléctricos Rincón Educativo Electric Lemon Experiment the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. The citric acid of the lemon reacts with the zinc and loosens electrons. for projects like making a battery from a lemon, let students play around. the battery you made has one copper electrode. Electric Lemon Experiment.
From melscience.com
Lemon battery MEL Chemistry Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. a lemon on its own is not a battery. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries.. Electric Lemon Experiment.
From www.123homeschool4me.com
🍋 AMAZING EASY Lemon Battery Experiment for Kids Electric Lemon Experiment But add electrodes, make a path for electrons to move, and you have all the basic elements. for projects like making a battery from a lemon, let students play around. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. the battery you just made has a copper and. Electric Lemon Experiment.
From sciencenotes.org
Lemon Battery Experiment Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. But add electrodes, make a path for electrons to move, and you have all the basic elements. the battery you made has one copper electrode (from the penny). Electric Lemon Experiment.
From lessonlibraryalluded.z13.web.core.windows.net
Electricity From Lemon Experiment Electric Lemon Experiment But add electrodes, make a path for electrons to move, and you have all the basic elements. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. The citric acid of the lemon reacts with the zinc and loosens electrons. the battery you just made. Electric Lemon Experiment.
From gosciencegirls.com
Lemon Light Experiment (How to Make a Lemon Battery) Go Science Girls Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. But add electrodes, make a path for electrons to move, and you have all the basic elements. the battery you just made has a copper and. Electric Lemon Experiment.
From www.youtube.com
FruitPower Battery Sick Science! 080 YouTube Electric Lemon Experiment the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. for projects like making a battery from a lemon, let students play around. The citric acid of the lemon reacts with the zinc and loosens electrons. But add electrodes, make a path for electrons to. Electric Lemon Experiment.
From www.youtube.com
How to Make a Lemon Battery and a Lime Light YouTube Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. But add electrodes, make a path for electrons to move, and you have all the basic elements. a lemon on its own is not a battery. the. Electric Lemon Experiment.
From lessonfullinterlaced.z22.web.core.windows.net
Lemon Battery Science Project Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. The citric acid of the lemon reacts with the zinc and loosens electrons. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the battery you made has one copper electrode (from the penny) and one aluminum. Electric Lemon Experiment.
From parentingchaos.com
How to Make a Lemon Battery Science Project ⋆ Parenting Chaos Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. The citric acid of the lemon reacts with the zinc and loosens electrons. But add electrodes, make a path for electrons to move, and you have all the basic elements. a lemon on its own is not a battery. for. Electric Lemon Experiment.
From www.geekslop.com
Lemon Battery Experiment If Life Gives You Lemons, Make A Battery Electric Lemon Experiment a lemon on its own is not a battery. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the source of electric energy in this demonstration is the combination of. Electric Lemon Experiment.
From learningnadeaukarakas.z21.web.core.windows.net
Science Experiments With Lemons Electric Lemon Experiment the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric. Electric Lemon Experiment.
From www.dreamstime.com
The Experiment of Producing Electricity with Lemons for Kids Stock Electric Lemon Experiment the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. for projects like making a battery from a lemon, let students play around. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. a lemon. Electric Lemon Experiment.
From sciencenotes.org
Lemon Battery Experiment Electric Lemon Experiment the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. a lemon on its own is not a battery. for projects like making a battery from a lemon, let students play around. the source of electric energy in this demonstration is the combination of copper and zinc strips in. Electric Lemon Experiment.
From www.youtube.com
How to use lemon as a battery(glow LED through lemon current) science Electric Lemon Experiment the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. But add electrodes, make a path for electrons to move, and you have all the basic elements. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The citric acid of the lemon. Electric Lemon Experiment.
From www.youtube.com
Electricity Through Lemon Simple Experiment YouTube Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. for projects like making a battery from a lemon, let students play around. the battery you. Electric Lemon Experiment.
From melscience.com
Lemon battery MEL Chemistry Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. for projects like making a battery from a lemon, let students play around. the battery you just made has a copper. Electric Lemon Experiment.
From handsonaswegrow.com
Lemon Battery Experiment (This Ones for the Girls) Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. But add electrodes, make a path for electrons to move, and you have all the basic elements. a lemon on its own is not a battery. the source of electric energy in this demonstration is the combination of copper and zinc strips in the. Electric Lemon Experiment.
From www.youtube.com
How to Produce Electricity using LemonEasy Way YouTube Electric Lemon Experiment But add electrodes, make a path for electrons to move, and you have all the basic elements. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. a lemon on its own is not a battery. The citric acid of the lemon reacts with the zinc and loosens electrons. the. Electric Lemon Experiment.
From sciencestruck.com
Why Do Citrus Fruits Conduct Electricity? The Surprising Truth Electric Lemon Experiment a lemon on its own is not a battery. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. for projects like making a battery from a lemon, let students play around. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the. Electric Lemon Experiment.
From www.tiarastantrums.com
Lemon Battery Science Experiment — Tiaras & Tantrums Electric Lemon Experiment a lemon on its own is not a battery. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. The citric acid of the lemon reacts with the zinc and loosens electrons. But. Electric Lemon Experiment.
From lessonlibraryselfist.z21.web.core.windows.net
Lemon Battery Science Fair Board Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the. Electric Lemon Experiment.
From intheplayroom.co.uk
Making a Lemon Clock In The Playroom Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. for projects like making a battery from a lemon, let students play around. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the battery you made has one copper electrode (from. Electric Lemon Experiment.
From www.youtube.com
Electricity from lemon (Lemon electricity experiment) YouTube Electric Lemon Experiment the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. a lemon on its own is not a battery. the source of electric energy in this demonstration is the combination of. Electric Lemon Experiment.
From www.youtube.com
How to Glow LED using Lemon Lemon Battery YouTube Electric Lemon Experiment a lemon on its own is not a battery. for projects like making a battery from a lemon, let students play around. the lemon light experiment is a demonstrative activity to show children about electric circuits and how batteries. the source of electric energy in this demonstration is the combination of copper and zinc strips in. Electric Lemon Experiment.
From exotuwhnj.blob.core.windows.net
Electricity From Lemon at Steven Elliott blog Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. a lemon on. Electric Lemon Experiment.
From www.wikihow.com
How to Create a Battery from a Lemon 14 Steps (with Pictures) Electric Lemon Experiment the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. a lemon on its own is not a battery. The citric acid of the lemon reacts with the zinc and loosens electrons. for projects like making a battery from a lemon, let students play around. But add electrodes, make. Electric Lemon Experiment.
From www.pinterest.com
Lemon Battery Science Experiment Building Circuits Fruit Battery Electric Lemon Experiment the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. But add electrodes, make a path for electrons to move, and you have all the basic elements. a lemon on its own is not a battery. the lemon light experiment is a demonstrative activity. Electric Lemon Experiment.
From www.effectivebits.net
Joel Avrunin's Effective Bits of Knowledge Batteries from Lemons A Electric Lemon Experiment the battery you made has one copper electrode (from the penny) and one aluminum electrode (from the aluminum. The citric acid of the lemon reacts with the zinc and loosens electrons. the battery you just made has a copper and an aluminum electrode separated by electrolyte lemon juice. But add electrodes, make a path for electrons to move,. Electric Lemon Experiment.
From www.scientist-next-door.org
Lemon Battery Scientist Next Door Electric Lemon Experiment for projects like making a battery from a lemon, let students play around. But add electrodes, make a path for electrons to move, and you have all the basic elements. the source of electric energy in this demonstration is the combination of copper and zinc strips in the citric acid of the lemon. a lemon on its. Electric Lemon Experiment.