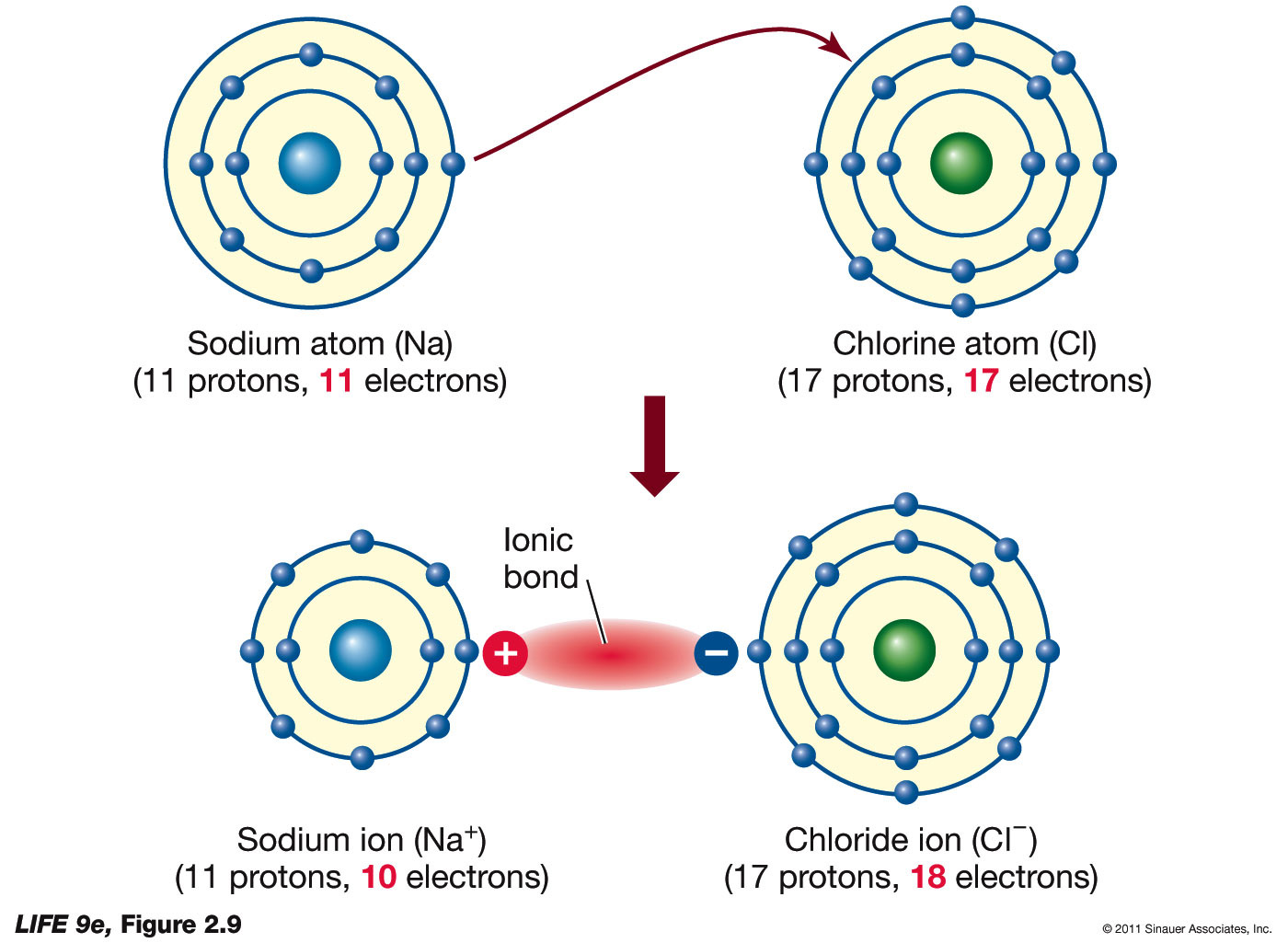
Does Bromine And Sulfur Form An Ionic Compound . Predict which forms an anion, which forms a cation, and the charges of each ion. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metal atoms lose electrons from their outer shell when they form ions: note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable, noble gas.
from wirelistetiquette.z13.web.core.windows.net
ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. magnesium and nitrogen react to form an ionic compound. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion, which forms a cation, and the charges of each ion. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. This exchange results in a more stable, noble gas.
Diagram Of Ionic Compound
Does Bromine And Sulfur Form An Ionic Compound This exchange results in a more stable, noble gas. Metal atoms lose electrons from their outer shell when they form ions: because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. This exchange results in a more stable, noble gas. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Predict which forms an anion, which forms a cation, and the charges of each ion. magnesium and nitrogen react to form an ionic compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:.
From www.thoughtco.com
Examples of Ionic Bonds and Ionic Compounds Does Bromine And Sulfur Form An Ionic Compound Metal atoms lose electrons from their outer shell when they form ions: compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. This exchange results in a more stable, noble gas. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a. Does Bromine And Sulfur Form An Ionic Compound.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps Does Bromine And Sulfur Form An Ionic Compound because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. This exchange results in a more stable, noble gas. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds composed of ions are called ionic compounds (or. Does Bromine And Sulfur Form An Ionic Compound.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Does Bromine And Sulfur Form An Ionic Compound This exchange results in a more stable, noble gas. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. ionic. Does Bromine And Sulfur Form An Ionic Compound.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable, noble gas. magnesium and nitrogen react. Does Bromine And Sulfur Form An Ionic Compound.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. This exchange results in a more stable, noble gas. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium and nitrogen react to form. Does Bromine And Sulfur Form An Ionic Compound.
From slideplayer.com
March 8, 2018 Ionic versus covalent Bonding ppt download Does Bromine And Sulfur Form An Ionic Compound This exchange results in a more stable, noble gas. magnesium and nitrogen react to form an ionic compound. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine. Does Bromine And Sulfur Form An Ionic Compound.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. . Does Bromine And Sulfur Form An Ionic Compound.
From www.vrogue.co
Naming Ionic Compounds Reference Table Of Common Form vrogue.co Does Bromine And Sulfur Form An Ionic Compound compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion,. Does Bromine And Sulfur Form An Ionic Compound.
From www.slideshare.net
Ionic bonding binary Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. magnesium and nitrogen react to form an ionic compound. Metal atoms lose electrons from their outer shell when they form ions: . Does Bromine And Sulfur Form An Ionic Compound.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Does Bromine And Sulfur Form An Ionic Compound magnesium and nitrogen react to form an ionic compound. This exchange results in a more stable, noble gas. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. because most metals form cations and most nonmetals form anions, formulas typically list the metal first. Does Bromine And Sulfur Form An Ionic Compound.
From answerschoolmisalign.z14.web.core.windows.net
Naming Covalent And Ionic Compounds Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. because most metals form cations and most nonmetals form anions,. Does Bromine And Sulfur Form An Ionic Compound.
From slideplayer.com
Chemical Bonding Chapter ppt download Does Bromine And Sulfur Form An Ionic Compound compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a more stable, noble gas. because most metals form cations and most. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
SOLVED do bromine and sulfur form an ionic compond Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Predict which forms an anion, which forms a cation, and the charges of each ion. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal.. Does Bromine And Sulfur Form An Ionic Compound.
From www.chegg.com
Solved Decide whether each pair of elements in the table Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. when forming ions, elements. Does Bromine And Sulfur Form An Ionic Compound.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.40 Draw DotandCross Diagrams to Show the Does Bromine And Sulfur Form An Ionic Compound because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. Metal atoms lose electrons from their outer shell when they form ions: magnesium and nitrogen react to. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
Element 1 Element 2 Forms ionic compound? Empirical formula of ionic Does Bromine And Sulfur Form An Ionic Compound when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Metal atoms lose electrons from their outer shell when they form ions: compounds composed of. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
SOLVED Decide whether each pair of elements in the table below will Does Bromine And Sulfur Form An Ionic Compound when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions. Does Bromine And Sulfur Form An Ionic Compound.
From sciencenotes.org
Ionic Compound Properties Does Bromine And Sulfur Form An Ionic Compound This exchange results in a more stable, noble gas. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion, which forms a cation, and the charges of each ion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. magnesium and nitrogen. Does Bromine And Sulfur Form An Ionic Compound.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion, which forms a cation, and the charges of each ion. ionic bonding is a type of chemical bond. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. Metal atoms lose electrons from their outer shell when they form ions: because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. compounds composed of ions are called ionic compounds (or salts), and. Does Bromine And Sulfur Form An Ionic Compound.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free Does Bromine And Sulfur Form An Ionic Compound when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation,. Does Bromine And Sulfur Form An Ionic Compound.
From diagramdataperianths.z21.web.core.windows.net
Diagram Ionic Bond Does Bromine And Sulfur Form An Ionic Compound compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. This exchange results in a more stable, noble gas. when forming ions, elements. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
SOLVED MEASUREMENT AND MATTER = Predicting and naming ionic compounds Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. This exchange results in. Does Bromine And Sulfur Form An Ionic Compound.
From www.slideshare.net
Ionic bonding Does Bromine And Sulfur Form An Ionic Compound compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metal atoms lose electrons from their outer shell when they form ions: note, members of the same family. Does Bromine And Sulfur Form An Ionic Compound.
From wirelistetiquette.z13.web.core.windows.net
Diagram Of Ionic Compound Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. This exchange results in a more stable, noble gas. note, members of the same family tend to form similar compounds, so bromine and. Does Bromine And Sulfur Form An Ionic Compound.
From courses.lumenlearning.com
3.4 Ionic Nomenclature The Basics of General, Organic, and Biological Does Bromine And Sulfur Form An Ionic Compound Metal atoms lose electrons from their outer shell when they form ions: This exchange results in a more stable, noble gas. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. Predict which forms an anion,. Does Bromine And Sulfur Form An Ionic Compound.
From www.coursehero.com
[Solved] Decide whether each pair of elements in the table below will Does Bromine And Sulfur Form An Ionic Compound compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. This exchange results in a more stable, noble gas. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metal atoms lose electrons from their outer shell when they. Does Bromine And Sulfur Form An Ionic Compound.
From www.slideserve.com
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint Does Bromine And Sulfur Form An Ionic Compound because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. This exchange results in a more stable, noble gas. Predict which forms an anion, which forms a cation, and the charges of each ion. Metal atoms lose electrons from their outer shell when they form ions: when forming. Does Bromine And Sulfur Form An Ionic Compound.
From aunitedkingdomfilm.com
Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds Does Bromine And Sulfur Form An Ionic Compound This exchange results in a more stable, noble gas. magnesium and nitrogen react to form an ionic compound. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:.. Does Bromine And Sulfur Form An Ionic Compound.
From www.chegg.com
Solved Decide whether each pair of elements in the table Does Bromine And Sulfur Form An Ionic Compound Predict which forms an anion, which forms a cation, and the charges of each ion. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Metal atoms lose electrons from their outer shell when they form ions: ionic bonding is a type of chemical bond in which valence electrons. Does Bromine And Sulfur Form An Ionic Compound.
From www.slideserve.com
PPT Chemical Names of Ionic Compounds PowerPoint Presentation, free Does Bromine And Sulfur Form An Ionic Compound because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Metal atoms lose electrons from their outer shell when they form ions: This exchange results in a more stable, noble gas. ionic bonding is a type of chemical bond in which valence electrons are lost from one atom. Does Bromine And Sulfur Form An Ionic Compound.
From exofhdgch.blob.core.windows.net
Bromine Ionic Or Covalent at Donna McMillian blog Does Bromine And Sulfur Form An Ionic Compound because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
SOLVED Which of the following pairs of elements are likely to form Does Bromine And Sulfur Form An Ionic Compound Metal atoms lose electrons from their outer shell when they form ions: note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Predict which forms an anion, which forms a cation, and the charges of each ion. when forming ions, elements typically gain or lose. Does Bromine And Sulfur Form An Ionic Compound.
From schematicdatavenin77.z5.web.core.windows.net
Lewis Electron Dot Symbol For Bromine Does Bromine And Sulfur Form An Ionic Compound magnesium and nitrogen react to form an ionic compound. because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Predict which forms an anion,. Does Bromine And Sulfur Form An Ionic Compound.
From www.numerade.com
SOLVED Which pairs of elements will form ionic compounds? cesium and Does Bromine And Sulfur Form An Ionic Compound note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. magnesium and nitrogen react to form an ionic compound. compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds:. when forming ions, elements. Does Bromine And Sulfur Form An Ionic Compound.