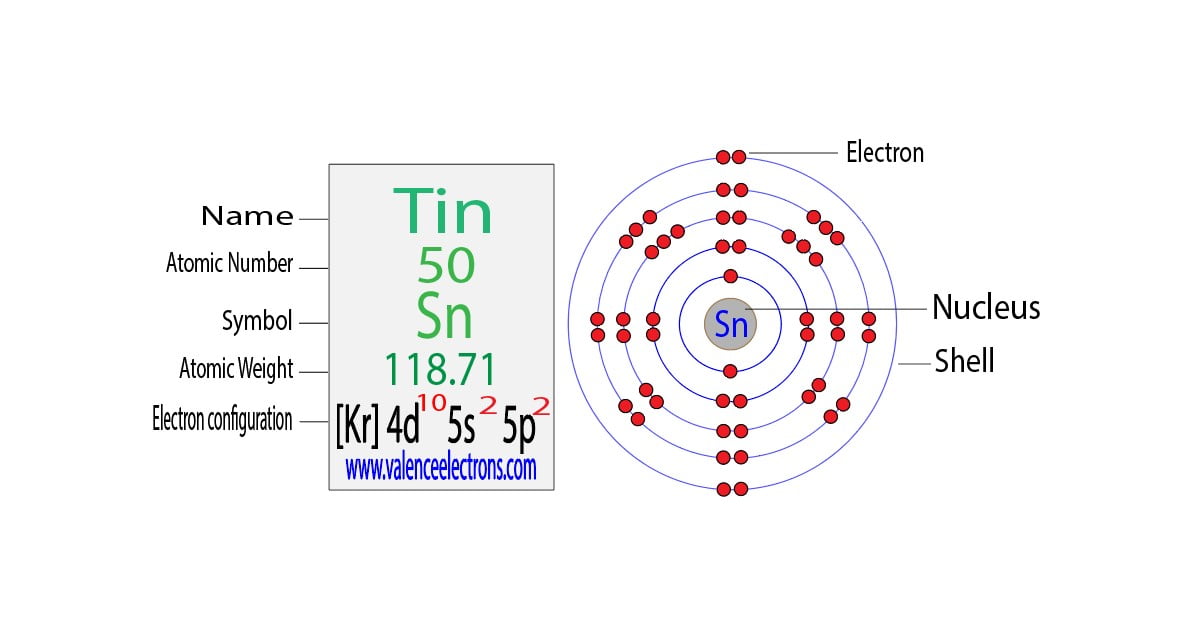
Tin Electron Orbital . The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Where, 5p 2 indicates that the 5p subshell has 2 electrons. There are multiple orbitals within an atom. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Each has its own specific energy level and properties.
from valenceelectrons.com
The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. There are multiple orbitals within an atom. Each has its own specific energy level and properties. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin.
Tin(Sn) electron configuration and orbital diagram
Tin Electron Orbital Each has its own specific energy level and properties. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Each has its own specific energy level and properties. There are multiple orbitals within an atom. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p.
From www.newtondesk.com
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6. Tin Electron Orbital.
From www.greatbigcanvas.com
5d electron orbitals Wall Art, Canvas Prints, Framed Prints, Wall Peels Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p. Tin Electron Orbital.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Electron Orbital Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. There are multiple orbitals within an atom. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Where, 5p 2 indicates that. Tin Electron Orbital.
From techdiagrammer.com
The Ultimate Guide to Understanding Electron Orbital Filling Diagrams Tin Electron Orbital The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. So the electron configuration will be 1s 2. Tin Electron Orbital.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Electron Orbital So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d. Tin Electron Orbital.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica Tin Electron Orbital Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Each has its own specific energy level and properties. Where, 5p 2 indicates that the 5p subshell has 2 electrons. There are multiple orbitals within an atom. The tin orbital diagram is a graphical representation of the electron configuration of the element tin. Tin Electron Orbital.
From sharihamish.blogspot.com
orbital diagram of tin ShariHamish Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. There are multiple orbitals within an atom. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Each has its own specific energy level and properties. So the. Tin Electron Orbital.
From electraschematics.com
Understanding the Tin Orbital Diagram A Complete Guide for Beginners Tin Electron Orbital Where, 5p 2 indicates that the 5p subshell has 2 electrons. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Tin Electron Orbital.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. The. Tin Electron Orbital.
From favpng.com
Aufbau Principle Molecular Orbital Diagram Electron Configuration Tin Electron Orbital Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Where, 5p 2 indicates that the 5p subshell has 2 electrons. There are multiple orbitals within an atom. The orbital diagram of tin shows that. Tin Electron Orbital.
From chemistrytalk.org
Orbital Diagrams ChemTalk Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Where,. Tin Electron Orbital.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Electron Orbital Where, 5p 2 indicates that the 5p subshell has 2 electrons. Each has its own specific energy level and properties. There are multiple orbitals within an atom. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Tin has. Tin Electron Orbital.
From www.youtube.com
Orbital Diagram Tin YouTube Tin Electron Orbital Where, 5p 2 indicates that the 5p subshell has 2 electrons. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2.. Tin Electron Orbital.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Electron Orbital Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Electrons exhibit a negative charge and are found. Tin Electron Orbital.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Electron Orbital Where, 5p 2 indicates that the 5p subshell has 2 electrons. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume. Tin Electron Orbital.
From www.youtube.com
Electron Configuration of Tin Sn Lesson YouTube Tin Electron Orbital Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Electronic orbitals are regions within the atom in which electrons have the highest probability. Tin Electron Orbital.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Electron Orbital Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. There are multiple orbitals within an atom. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined. Tin Electron Orbital.
From www.alamy.com
3d electron orbitals, computer model. An electron orbital is a region Tin Electron Orbital The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Where, 5p 2 indicates that the 5p subshell. Tin Electron Orbital.
From www.alamy.com
4f electron orbitals, general set, computer model. An electron orbital Tin Electron Orbital There are multiple orbitals within an atom. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Electrons exhibit a. Tin Electron Orbital.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Electron Orbital The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Electronic orbitals are regions within the atom in which electrons have. Tin Electron Orbital.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Electron Orbital There are multiple orbitals within an atom. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the. Tin Electron Orbital.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Tin Electron Orbital Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which. Tin Electron Orbital.
From valenceelectrons.com
Tin(Sn) electron configuration and orbital diagram Tin Electron Orbital The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. Each has its own specific energy level and properties. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2. Tin Electron Orbital.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Electron Orbital Where, 5p 2 indicates that the 5p subshell has 2 electrons. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. There are multiple orbitals within an atom. Each has its own specific energy level and properties.. Tin Electron Orbital.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Each. Tin Electron Orbital.
From www.shutterstock.com
Table Showing Electron Orbital Configuration Smallest Stock Vector Tin Electron Orbital Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. There are multiple orbitals within an atom. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Each. Tin Electron Orbital.
From aurorecollette.blogspot.com
orbital diagram of tin AuroreCollette Tin Electron Orbital Electronic orbitals are regions within the atom in which electrons have the highest probability of being found. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin. Each has its own specific energy level and. Tin Electron Orbital.
From chem.libretexts.org
6.6 The Shapes of Atomic Orbitals Chemistry LibreTexts Tin Electron Orbital The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Where, 5p 2 indicates that the 5p subshell has 2 electrons. Electronic orbitals are regions within the atom in which electrons have the highest probability of being. Tin Electron Orbital.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Tin Electron Orbital The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Where, 5p 2 indicates that the 5p subshell has 2 electrons. The tin orbital diagram is a graphical representation of the electron configuration of the element tin. Tin Electron Orbital.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Tin Electron Orbital Each has its own specific energy level and properties. The tin orbital diagram is a graphical representation of the electron configuration of the element tin (sn) on the periodic table. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Where, 5p. Tin Electron Orbital.
From www.youtube.com
Full and Abbreviated Electron Configuration of Ruthenium Ru YouTube Tin Electron Orbital The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Where, 5p 2 indicates that the 5p subshell has 2 electrons. So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6. Tin Electron Orbital.
From schematicfixpulpits.z21.web.core.windows.net
Electron Configuration Energy Level Diagram Tin Electron Orbital So the electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can. Tin Electron Orbital.
From www.alamy.es
Los electrones orbitales 3d, el modelo de la computadora. Un electrón Tin Electron Orbital Electrons exhibit a negative charge and are found around the nucleus of the atom in electron orbitals, defined as the volume of space in which the electron can be found within. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p. Tin Electron Orbital.
From scienceinfo.com
Electron Orbitals Tin Electron Orbital There are multiple orbitals within an atom. Each has its own specific energy level and properties. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. The tin orbital diagram is a graphical representation of the electron. Tin Electron Orbital.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Tin Electron Orbital Each has its own specific energy level and properties. There are multiple orbitals within an atom. The orbital diagram of tin shows that the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, the 3s subshell has 2 electrons, the 3p. Electronic orbitals are regions within the atom in which electrons have. Tin Electron Orbital.