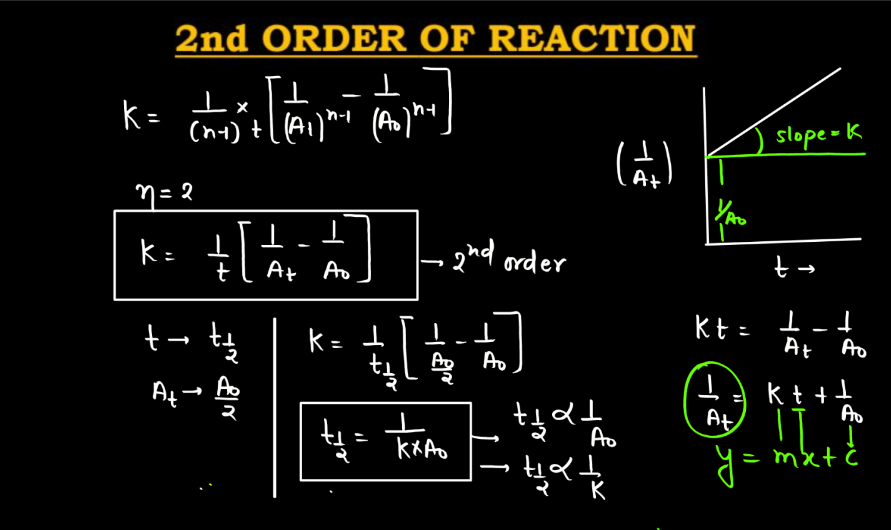
Sequence Of Reaction Meaning . Molecularity is the number of reacting. Describes how the concentration of reactants affects the rate. It is the sum of the exponents of. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. That is to say, \(\left[.
from www.careerpower.in
Describes how the concentration of reactants affects the rate. It is the sum of the exponents of. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. Molecularity is the number of reacting. That is to say, \(\left[. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what.
Order of Reaction Definition, Types, Formula and Methods
Sequence Of Reaction Meaning The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. It is the sum of the exponents of. Describes how the concentration of reactants affects the rate. That is to say, \(\left[. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Molecularity is the number of reacting.
From www.researchgate.net
Sequence of reactions leading to compounds 4 synthesis. Download Sequence Of Reaction Meaning It is the sum of the exponents of. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Describes how the concentration of reactants affects the rate. That is to say, \(\left[. Molecularity is the number of reacting. The order of a reaction refers to the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Molecularity is the number of reacting. That is to say, \(\left[. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Describes how the. Sequence Of Reaction Meaning.
From www.toppr.com
In the following sequence of reactions. The compound C formed will be Sequence Of Reaction Meaning That is to say, \(\left[. Describes how the concentration of reactants affects the rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Molecularity is the number of reacting. It is the. Sequence Of Reaction Meaning.
From www.thesciencehive.co.uk
The Reactivity Series* — the science sauce Sequence Of Reaction Meaning That is to say, \(\left[. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of. Sequence Of Reaction Meaning.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 3 Chemistry Learner Sequence Of Reaction Meaning In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. It is the sum of the exponents of. Order of a reaction is the. Sequence Of Reaction Meaning.
From www.youtube.com
Reaction Order for YouTube Sequence Of Reaction Meaning More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a reaction refers to the relationship between the reactants’ concentrations and. Sequence Of Reaction Meaning.
From slidesharenow.blogspot.com
Overall Order Of Reaction slideshare Sequence Of Reaction Meaning Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. That is to say, \(\left[. Describes how the concentration of reactants affects the rate. Molecularity is the number of reacting. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Chapter 11 Rate of Reaction PowerPoint Presentation, free Sequence Of Reaction Meaning Describes how the concentration of reactants affects the rate. That is to say, \(\left[. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Molecularity is the number of reacting. The order of a chemical reaction is defined as the sum of the powers of the concentration of. Sequence Of Reaction Meaning.
From www.showme.com
Order of a reaction Science, Chemistry, Rates Of Reaction ShowMe Sequence Of Reaction Meaning That is to say, \(\left[. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. The order of a reaction refers to the relationship. Sequence Of Reaction Meaning.
From www.chegg.com
For this sequence of reactions, draw the major Sequence Of Reaction Meaning The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. That is to say, \(\left[. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. Molecularity is the number of reacting. More specifically, the. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Chemical Rate Laws ORDER OF REACTION PowerPoint Sequence Of Reaction Meaning Molecularity is the number of reacting. It is the sum of the exponents of. That is to say, \(\left[. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. Order of. Sequence Of Reaction Meaning.
From chemistrytalk.org
Chemical Reactions Infographic ChemTalk Sequence Of Reaction Meaning The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. That is to say, \(\left[. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. Molecularity is the number of reacting. More specifically, the. Sequence Of Reaction Meaning.
From testbook.com
Order of Reaction Learn Definition, Types, Determination, Values Sequence Of Reaction Meaning That is to say, \(\left[. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. Describes how the concentration of reactants affects the rate. Molecularity is the number of. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Chapter Five Rates of Chemical Reaction PowerPoint Presentation Sequence Of Reaction Meaning It is the sum of the exponents of. Describes how the concentration of reactants affects the rate. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. That is to say, \(\left[. The order of a chemical reaction is defined as the sum of the powers of the concentration of the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning Molecularity is the number of reacting. Describes how the concentration of reactants affects the rate. That is to say, \(\left[. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Reaction order PowerPoint Presentation ID834824 Sequence Of Reaction Meaning Describes how the concentration of reactants affects the rate. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. That is to say, \(\left[. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. Molecularity is the number of. Sequence Of Reaction Meaning.
From www.thoughtco.com
Types of Chemical Reactions (With Examples) Sequence Of Reaction Meaning It is the sum of the exponents of. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. That is to say,. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning Describes how the concentration of reactants affects the rate. Molecularity is the number of reacting. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. That is to say, \(\left[. It is the sum of the exponents of. The order of a reaction refers to the relationship between. Sequence Of Reaction Meaning.
From www.studypool.com
SOLUTION Order of reaction definition and zero order reaction Studypool Sequence Of Reaction Meaning In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Molecularity is the number of reacting. The order of a chemical reaction is defined. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Order Of Reaction PowerPoint Presentation, free download ID Sequence Of Reaction Meaning Molecularity is the number of reacting. It is the sum of the exponents of. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the. Sequence Of Reaction Meaning.
From pediaa.com
Difference Between Molecularity and Order of Reaction Definition Sequence Of Reaction Meaning The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. It is the sum of the exponents of. Describes. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Order Of Reaction PowerPoint Presentation, free download ID Sequence Of Reaction Meaning Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. That is to say, \(\left[. It is the sum of the exponents of. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. More specifically, the reaction order is the exponent to which the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning Molecularity is the number of reacting. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. That is to say, \(\left[. Order of a reaction is the sum of. Sequence Of Reaction Meaning.
From www.chemistrylearner.com
Firstorder Reaction Definition, Examples, and Equations Sequence Of Reaction Meaning It is the sum of the exponents of. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. That is to say, \(\left[. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Order. Sequence Of Reaction Meaning.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps Sequence Of Reaction Meaning The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Describes how the concentration of reactants affects the rate. More specifically, the reaction order is the exponent to which. Sequence Of Reaction Meaning.
From www.youtube.com
Order of Reaction and their Determination YouTube Sequence Of Reaction Meaning It is the sum of the exponents of. That is to say, \(\left[. Describes how the concentration of reactants affects the rate. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a chemical reaction is defined as the sum of the powers. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Order Of Reaction PowerPoint Presentation, free download ID Sequence Of Reaction Meaning Describes how the concentration of reactants affects the rate. Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a reaction refers to the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning That is to say, \(\left[. Molecularity is the number of reacting. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. More specifically, the. Sequence Of Reaction Meaning.
From www.studypool.com
SOLUTION Order of reaction definition and zero order reaction Studypool Sequence Of Reaction Meaning Molecularity is the number of reacting. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Describes how the concentration of reactants affects the rate. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. It is the sum. Sequence Of Reaction Meaning.
From www.coursehero.com
[Solved] 6. Consider the sequence of reactions shown below Reaction Sequence Of Reaction Meaning In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in the rate equation of that particular chemical reaction. Order of a reaction is the sum. Sequence Of Reaction Meaning.
From www.biologyonline.com
Firstorder Definition and Examples Biology Online Dictionary Sequence Of Reaction Meaning The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. It is the sum of the exponents of. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. More specifically, the reaction order is the exponent to which the. Sequence Of Reaction Meaning.
From www.careerpower.in
Order of Reaction Definition, Types, Formula and Methods Sequence Of Reaction Meaning Describes how the concentration of reactants affects the rate. That is to say, \(\left[. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly proportional to the concentration of \(\ce{a}\) raised to the first power. Molecularity is the number of reacting. The order of a chemical reaction is defined as the sum of the powers of the. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Reaction Mechanisms Steps of a Reaction PowerPoint Presentation Sequence Of Reaction Meaning More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. The order of a reaction refers to the relationship between the reactants’ concentrations and the reaction rate. The order of a chemical reaction is defined as the sum of the powers of the concentration of the reactants in. Sequence Of Reaction Meaning.
From www.slideserve.com
PPT Reaction order PowerPoint Presentation, free download ID834824 Sequence Of Reaction Meaning Order of a reaction is the sum of the coefficients of the reacting species involved in the rate equation. More specifically, the reaction order is the exponent to which the concentration of that species is raised, and it indicates to what. Molecularity is the number of reacting. In the reaction \(\ce{a} \rightarrow \ce{b}\), the rate of the reaction is directly. Sequence Of Reaction Meaning.