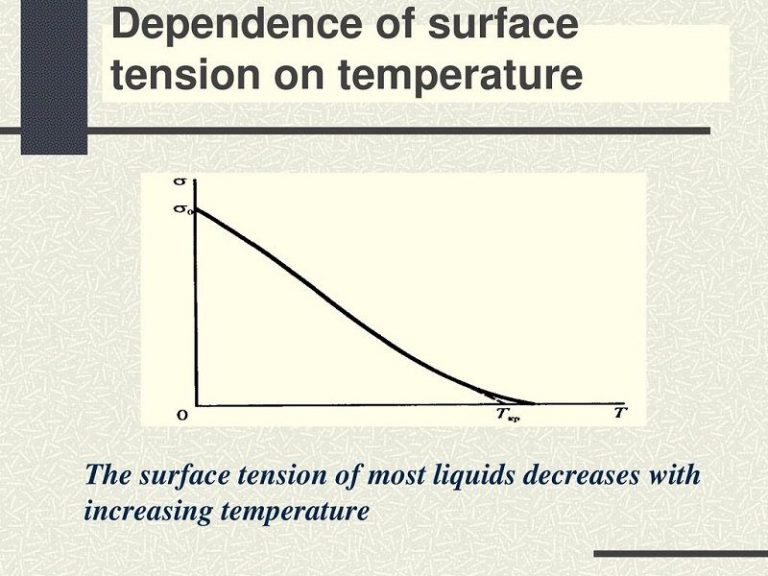
Water Surface Tension Versus Temperature . In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with temperature as. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of the surrounding.
from studiousguy.com
The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of the surrounding. It would take a force of 72 dynes to break a surface film of water 1 cm long. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The surface tension of water is 72 dynes/cm at 25°c.
10 Surface Tension Examples in Daily Life StudiousGuy
Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The surface tension of water is 72 dynes/cm at 25°c. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. It would take a force of 72 dynes to break a surface film of water 1 cm long. The influence of the surrounding. The surface tension of water decreases significantly with temperature as.
From www.researchgate.net
Figure A.2 Variation of the surface tension versus temperature [15 Water Surface Tension Versus Temperature It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The surface tension of water decreases significantly with temperature as. Water has a. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension versus temperature for water in contact with air Water Surface Tension Versus Temperature The influence of the surrounding. It would take a force of 72 dynes to break a surface film of water 1 cm long. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The surface tension of water decreases significantly with temperature as. The international association for the properties of steam. Water Surface Tension Versus Temperature.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The surface tension of water decreases significantly with temperature as. The influence of the surrounding. The international association for the properties. Water Surface Tension Versus Temperature.
From www.researchgate.net
Equilibrium surface tension of MIBC in 5 M NaCl and 4 M KCl solutions Water Surface Tension Versus Temperature The surface tension of water is 72 dynes/cm at 25°c. The influence of the surrounding. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. In. Water Surface Tension Versus Temperature.
From mavink.com
Water Surface Tension Chart Water Surface Tension Versus Temperature The surface tension of water decreases significantly with temperature as. The influence of the surrounding. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. From. Water Surface Tension Versus Temperature.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Water Surface Tension Versus Temperature Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. The influence of the surrounding. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to. Water Surface Tension Versus Temperature.
From www.doubtnut.com
Which graph represents the variation of surface tension with temperatu Water Surface Tension Versus Temperature It would take a force of 72 dynes to break a surface film of water 1 cm long. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of. Water Surface Tension Versus Temperature.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water Surface Tension Versus Temperature From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids.. Water Surface Tension Versus Temperature.
From www.youtube.com
What is Effect Of Temperature on surface tension Properties of Water Surface Tension Versus Temperature The influence of the surrounding. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential. Water Surface Tension Versus Temperature.
From www.researchgate.net
Relationship between temperature and viscosity of liquid water Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The surface tension of water decreases significantly with temperature as. The surface tension of water is 72 dynes/cm at 25°c. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a). Water Surface Tension Versus Temperature.
From www.youtube.com
Surface Tension of Water Explained YouTube Water Surface Tension Versus Temperature It would take a force of 72 dynes to break a surface film of water 1 cm long. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of. Water Surface Tension Versus Temperature.
From www.mdpi.com
Liquids Free FullText Correlation of Surface Tension of Mono Water Surface Tension Versus Temperature From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. It would take a force of 72 dynes to break a surface film of water 1 cm long. The influence of the surrounding. The international association for the properties of steam (laps) has approved an international table of values for. Water Surface Tension Versus Temperature.
From www.researchgate.net
Variation of surface tension versus temperature for and Water Surface Tension Versus Temperature From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids.. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension as a function of temperature for 5 butanol in water Water Surface Tension Versus Temperature The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with temperature as. In general, surface tension decreases when temperature increases. Water Surface Tension Versus Temperature.
From www.researchgate.net
Calculated surface tension of wateroil with respect to temperature Water Surface Tension Versus Temperature Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The surface tension of water decreases significantly with temperature as. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. From a more theoretical perspective, the. Water Surface Tension Versus Temperature.
From www.hexahedron999.com
12. L’eau structurée et la tension de surface Hexahedron 999 Eau Water Surface Tension Versus Temperature Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of the surrounding. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. In general, surface tension decreases when temperature increases because cohesive forces. Water Surface Tension Versus Temperature.
From www.researchgate.net
The dependences of contact angle, viscosity and surface tension vs Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. It would take a force of 72 dynes to break a surface film of water 1 cm long. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water. Water Surface Tension Versus Temperature.
From www.researchgate.net
The dependence of surface tension on temperature for three common Water Surface Tension Versus Temperature The influence of the surrounding. The surface tension of water is 72 dynes/cm at 25°c. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. Water. Water Surface Tension Versus Temperature.
From manualdefecation.z21.web.core.windows.net
How To Explain Surface Tension Water Surface Tension Versus Temperature The surface tension of water is 72 dynes/cm at 25°c. The influence of the surrounding. It would take a force of 72 dynes to break a surface film of water 1 cm long. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. Water has a high surface tension (72.8 mn·m. Water Surface Tension Versus Temperature.
From www.researchgate.net
Variation of surface tension with temperature of fluidmedium pairs Water Surface Tension Versus Temperature The influence of the surrounding. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. It would take a force of 72 dynes to break a surface film of water 1. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface Tension versus temperature for the pure NaCl system at room Water Surface Tension Versus Temperature The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The influence of the surrounding. The surface tension of water decreases significantly with temperature as. The surface tension of water is 72 dynes/cm at 25°c. From a more theoretical perspective, the temperature dependence of contact angle. Water Surface Tension Versus Temperature.
From www.researchgate.net
Relationship between temperature and surface tension of liquid water Water Surface Tension Versus Temperature From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The surface tension of water decreases significantly with temperature as. In general, surface tension decreases when. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension as a function of the temperature. Download Scientific Water Surface Tension Versus Temperature The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of the surrounding. The international association for the properties of steam (laps) has approved an international table of. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension, temperature, weight of an open water bottle (purple Water Surface Tension Versus Temperature The influence of the surrounding. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to. Water Surface Tension Versus Temperature.
From www.researchgate.net
(a) The surface tension (i.e., interfacial tension with vacuum) of Water Surface Tension Versus Temperature The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases significantly with temperature as. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The influence of. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension and its derivative with respect to temperature of water Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8. Water Surface Tension Versus Temperature.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Water Surface Tension Versus Temperature The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The influence of the surrounding. Water has a high surface tension (72.8 mn·m −1 at 293.15. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension versus temperature for water in contact with air Water Surface Tension Versus Temperature The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. From a more theoretical perspective, the temperature dependence of contact angle of. Water Surface Tension Versus Temperature.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Water Surface Tension Versus Temperature The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The influence of the surrounding. The surface tension of water is 72 dynes/cm at 25°c. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension versus temperature for 0.2 wt heptanol solution and Water Surface Tension Versus Temperature The influence of the surrounding. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The surface tension of water decreases significantly with temperature as. It would. Water Surface Tension Versus Temperature.
From byjus.com
Explain the surface tension phenomenon with examples. Water Surface Tension Versus Temperature It would take a force of 72 dynes to break a surface film of water 1 cm long. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The influence. Water Surface Tension Versus Temperature.
From www.quora.com
Does temperature increase surface tension? Quora Water Surface Tension Versus Temperature The surface tension of water decreases significantly with temperature as. The surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. The international. Water Surface Tension Versus Temperature.
From www.researchgate.net
Changes in the ratio of water surface tension to density (s/r) vs Water Surface Tension Versus Temperature The international association for the properties of steam (laps) has approved an international table of values for the surface tension (a) of water in. The surface tension of water is 72 dynes/cm at 25°c. In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The influence of the surrounding. The surface. Water Surface Tension Versus Temperature.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Surface Tension Versus Temperature The influence of the surrounding. The surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases significantly with temperature as. Water has a high surface tension (72.8 mn·m −1 at 293.15 k [1]) compared to that of most other liquids. It would take a force of 72 dynes to break a surface film of water. Water Surface Tension Versus Temperature.
From www.researchgate.net
Surface tension versus temperature. Download Scientific Diagram Water Surface Tension Versus Temperature In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The influence of the surrounding. The surface tension of water decreases significantly with temperature as. From a more theoretical perspective, the temperature dependence of contact angle of water is also essential to estimation of several. The surface tension of water is. Water Surface Tension Versus Temperature.