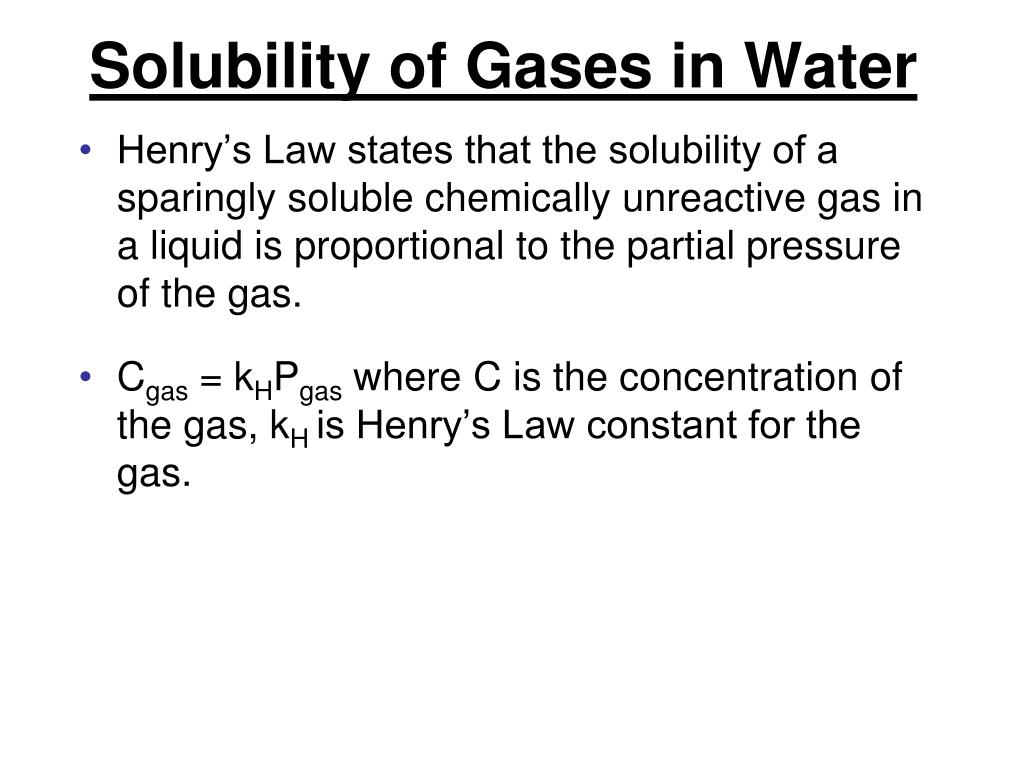
Which Gas Is More Soluble In Water . carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Easily liquifiable gases (co 2,. hydrogen chloride (hcl) gas is highly soluble in water. Due to hydrogen bonding with water, it is most soluble in water and is. Factors affecting the solubility of gases in liquids. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. ammonia is a colorless gas with a chemical formula nh 3. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the.
from www.slideserve.com
ammonia is a colorless gas with a chemical formula nh 3. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. Due to hydrogen bonding with water, it is most soluble in water and is. Factors affecting the solubility of gases in liquids. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. hydrogen chloride (hcl) gas is highly soluble in water. Easily liquifiable gases (co 2,. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging.
PPT Chapter 11 PowerPoint Presentation, free download ID5852920
Which Gas Is More Soluble In Water Due to hydrogen bonding with water, it is most soluble in water and is. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. Due to hydrogen bonding with water, it is most soluble in water and is. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. ammonia is a colorless gas with a chemical formula nh 3. Easily liquifiable gases (co 2,. hydrogen chloride (hcl) gas is highly soluble in water. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. Factors affecting the solubility of gases in liquids. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the.
From www.youtube.com
Solubility of Gases in Water (O2, N2, etc.) YouTube Which Gas Is More Soluble In Water Easily liquifiable gases (co 2,. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. hydrogen chloride (hcl) gas is highly soluble in water. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. carbon dioxide is weakly soluble in water, therefore it separates. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Which Gas Is More Soluble In Water Easily liquifiable gases (co 2,. ammonia is a colorless gas with a chemical formula nh 3. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Factors affecting the solubility. Which Gas Is More Soluble In Water.
From www.researchgate.net
Gas solubility data in pure water. Download Table Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. hydrogen chloride (hcl) gas is highly soluble in water. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. Easily liquifiable. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. Factors affecting the solubility of gases in liquids. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. hydrogen chloride (hcl) gas is highly soluble in water. thus, for example, the solubility of ammonia in water increases. Which Gas Is More Soluble In Water.
From www.studypool.com
SOLUTION Solved a soluble gas is absorbed in water using a packed Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. hydrogen chloride (hcl) gas is highly soluble in water. Factors affecting the solubility of gases in liquids. this. Which Gas Is More Soluble In Water.
From www.youtube.com
6 Gas Solubility YouTube Which Gas Is More Soluble In Water according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. ammonia is a colorless gas with a chemical formula nh 3. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. Easily liquifiable gases (co 2,. . Which Gas Is More Soluble In Water.
From www.doubtnut.com
Solubility of oxygen gas in water follows Henry's law. When the solubi Which Gas Is More Soluble In Water thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. Due to hydrogen bonding with water, it is most soluble in water and is. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. this would explain why $\ce{br_2}$ has. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Factors Affecting Solubility PowerPoint Presentation, free Which Gas Is More Soluble In Water carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. Due to hydrogen bonding with water, it is most soluble in water and is. this would explain why $\ce{br_2}$ has. Which Gas Is More Soluble In Water.
From www.youtube.com
Can Gases Dissolve in Water? YouTube Which Gas Is More Soluble In Water Due to hydrogen bonding with water, it is most soluble in water and is. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. Easily liquifiable gases (co 2,. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. ammonia is a colorless gas with. Which Gas Is More Soluble In Water.
From www.youtube.com
Solubility Curve Intro How to Read It Saturation Gases vs Solids Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. Factors affecting the solubility of gases in liquids. Due to hydrogen bonding with water, it is most soluble in water and is. Easily liquifiable gases (co 2,. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. according. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID3105812 Which Gas Is More Soluble In Water this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. hydrogen chloride (hcl) gas is highly soluble in water. Easily liquifiable gases (co 2,. Due to hydrogen bonding with water, it is most soluble in. Which Gas Is More Soluble In Water.
From www.ck12.org
Gas Solubility (use graphs) Overview ( Video ) Chemistry CK12 Which Gas Is More Soluble In Water hydrogen chloride (hcl) gas is highly soluble in water. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. Due to hydrogen bonding with water, it is most soluble in water and is. Easily liquifiable gases (co 2,. this would explain why $\ce{br_2}$ has a better solubility than. Which Gas Is More Soluble In Water.
From courses.lumenlearning.com
Gas Solubility and Temperature Introduction to Chemistry Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. ammonia is a colorless gas with a chemical formula nh 3. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly.. Which Gas Is More Soluble In Water.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. hydrogen chloride (hcl) gas is highly soluble in water. online calculator, figures. Which Gas Is More Soluble In Water.
From www.researchgate.net
The solubility curves for the noble gases according to water Which Gas Is More Soluble In Water hydrogen chloride (hcl) gas is highly soluble in water. ammonia is a colorless gas with a chemical formula nh 3. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID5581895 Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. Due to hydrogen bonding with water, it is most soluble in water and is. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. Easily liquifiable gases (co 2,. . Which Gas Is More Soluble In Water.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Oxygen Gas Solubility In Water Which Gas Is More Soluble In Water thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. ammonia is a colorless gas with a chemical formula nh 3. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. online calculator, figures and tables showing dynamic (absolute). Which Gas Is More Soluble In Water.
From rayb78.github.io
Solubility In Water Chart Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. ammonia is a colorless gas with a chemical formula nh 3. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. carbon dioxide is weakly soluble in water, therefore it separates into a gas. Which Gas Is More Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Which Gas Is More Soluble In Water Easily liquifiable gases (co 2,. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. ammonia is a colorless gas with a chemical formula nh 3. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. Factors affecting the solubility of gases in liquids. . Which Gas Is More Soluble In Water.
From mungfali.com
Gas Solubility Curve Which Gas Is More Soluble In Water Easily liquifiable gases (co 2,. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. Due to hydrogen bonding with water, it is most soluble in water and is. . Which Gas Is More Soluble In Water.
From www.scienceabc.com
Why Do Bubbles Form In A Glass Of Water That's Left Out? » ScienceABC Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Factors affecting the solubility of gases in. Which Gas Is More Soluble In Water.
From solutionpharmacy.in
Solubility of Gas in Liquids Solution Parmacy Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. hydrogen chloride (hcl) gas is highly soluble in water. Easily liquifiable gases (co 2,. Factors affecting the solubility of gases in liquids. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than. Which Gas Is More Soluble In Water.
From askfilo.com
Which gas is most soluble in water? Filo Which Gas Is More Soluble In Water this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. Easily liquifiable gases (co 2,. Factors affecting the solubility of gases in liquids. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of. Which Gas Is More Soluble In Water.
From www.toppr.com
(i) Gas (A) is more soluble in water than gas (B) at the same Which Gas Is More Soluble In Water this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. according to henry’s law, for an. Which Gas Is More Soluble In Water.
From methanegaskimawashi.blogspot.com
Methane Gas Solubility Of Methane Gas In Water Which Gas Is More Soluble In Water Factors affecting the solubility of gases in liquids. Due to hydrogen bonding with water, it is most soluble in water and is. according to henry’s law, for an ideal solution the solubility, c g, of a gas (1.38 × 10 −3 mol l −1, in this case) is directly. hydrogen chloride (hcl) gas is highly soluble in water.. Which Gas Is More Soluble In Water.
From methanegaskimawashi.blogspot.com
Methane Gas Solubility Of Methane Gas In Water Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. ammonia is a colorless gas with a chemical formula nh 3. hydrogen chloride (hcl) gas is highly soluble in water. Due to hydrogen bonding with water, it is most soluble in water and is. Factors affecting the solubility. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Chapter 9 Solutions PowerPoint Presentation, free download ID Which Gas Is More Soluble In Water hydrogen chloride (hcl) gas is highly soluble in water. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Due to hydrogen bonding with water, it is most soluble in. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID5852920 Which Gas Is More Soluble In Water thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. ammonia is a colorless gas with a chemical formula nh 3. Factors affecting the solubility of gases in liquids. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging.. Which Gas Is More Soluble In Water.
From www.doubtnut.com
Which noble gas is more soluble in water Which Gas Is More Soluble In Water this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Factors affecting the solubility of gases in liquids. ammonia is a colorless gas with a chemical formula nh 3. Easily liquifiable gases (co 2,. according. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Which Gas Is More Soluble In Water this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. Factors affecting the solubility of gases in liquids. carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Due to hydrogen bonding with water, it is most soluble in water and is. Easily liquifiable gases (co 2,.. Which Gas Is More Soluble In Water.
From jaedengokemcknight.blogspot.com
Under Which Conditions Will Gases Best Dissolve in Liquids Which Gas Is More Soluble In Water Easily liquifiable gases (co 2,. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. . Which Gas Is More Soluble In Water.
From www.doubtnut.com
At a given temperature, oxygen gas is more soluble in water than Nitro Which Gas Is More Soluble In Water online calculator, figures and tables showing dynamic (absolute) and kinematic viscosity of gasous and liquid ammonia at temperatures ranging. thus, for example, the solubility of ammonia in water increases more rapidly with increasing pressure than predicted by the. Due to hydrogen bonding with water, it is most soluble in water and is. Factors affecting the solubility of gases. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Which Gas Is More Soluble In Water hydrogen chloride (hcl) gas is highly soluble in water. Due to hydrogen bonding with water, it is most soluble in water and is. ammonia is a colorless gas with a chemical formula nh 3. Easily liquifiable gases (co 2,. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. online calculator, figures and. Which Gas Is More Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Which Gas Is More Soluble In Water carbon dioxide is weakly soluble in water, therefore it separates into a gas when the pressure is released. Easily liquifiable gases (co 2,. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. hydrogen chloride (hcl) gas is highly soluble in water. thus, for example, the solubility of ammonia in water increases more. Which Gas Is More Soluble In Water.
From www.numerade.com
SOLVED Hydrogen (H2) and ethane (C2H6) are both gases at room Which Gas Is More Soluble In Water ammonia is a colorless gas with a chemical formula nh 3. Due to hydrogen bonding with water, it is most soluble in water and is. this would explain why $\ce{br_2}$ has a better solubility than $\ce{o_2}$ in water. hydrogen chloride (hcl) gas is highly soluble in water. thus, for example, the solubility of ammonia in water. Which Gas Is More Soluble In Water.