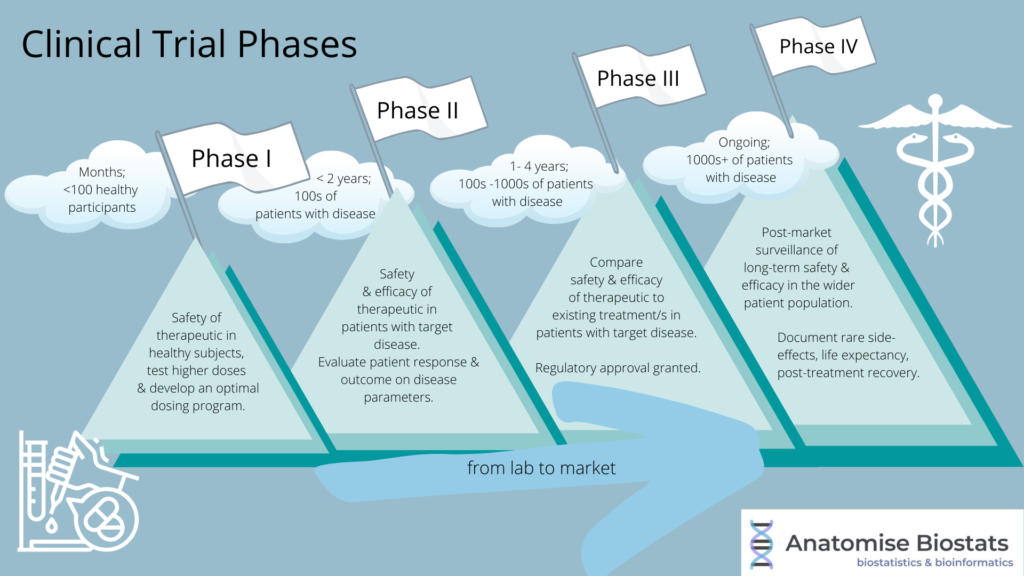
Drug Testing Phases . Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. They allow the safety and effectiveness of new drugs or treatments to be properly. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Find out the differences between. Let’s take a closer look at each stage to better understand what goes into early clinical. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Clinical trials and their individual phases are a very important part of clinical research. The drug is usually approved for use in. Phase 1 is the first testing in humans, primarily to test.
from anatomisebiostats.com
Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. They allow the safety and effectiveness of new drugs or treatments to be properly. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Let’s take a closer look at each stage to better understand what goes into early clinical. Find out the differences between. Clinical trials and their individual phases are a very important part of clinical research. The drug is usually approved for use in. Phase 1 is the first testing in humans, primarily to test. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial.
Clinical Trial Phases in Drug Development Biostatistics
Drug Testing Phases Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. They allow the safety and effectiveness of new drugs or treatments to be properly. Let’s take a closer look at each stage to better understand what goes into early clinical. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Phase 1 is the first testing in humans, primarily to test. Clinical trials and their individual phases are a very important part of clinical research. The drug is usually approved for use in. Find out the differences between. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average.
From gene.vision
Clinical trials Gene Vision Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Find out the differences between. Learn about the different stages of clinical testing of a new drug, from phase. Drug Testing Phases.
From www.dementiablog.org
The Global Clinical Trials Fund Alzheimer's Research UK Blog Drug Testing Phases Clinical trials and their individual phases are a very important part of clinical research. Find out the differences between. Let’s take a closer look at each stage to better understand what goes into early clinical. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Phase 1 is. Drug Testing Phases.
From discover.hubpages.com
How are drugs developed and approved? The drug development process Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. The drug is usually approved for use in. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Find out the differences between. The entire process of moving a drug from design to clinical trials takes 10. Drug Testing Phases.
From www.ildcollaborative.org
Phase IV IPF Clinical Trials ILD Collaborative Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Find out the differences between. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. The drug is usually. Drug Testing Phases.
From news.joindementiaresearch.nihr.ac.uk
Why a diabetes drug may offer potential treatment for Alzheimer’s Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. They allow the safety and effectiveness of new drugs or treatments to be properly. Phase 1 is the first. Drug Testing Phases.
From www.slideteam.net
New Drug Development Process Flowchart For Clinical Trial Phases With Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Clinical trials and their individual phases are a very important part of clinical research. They allow the safety and effectiveness of new drugs or treatments to be properly. Learn what phases of clinical trials are, how they are. Drug Testing Phases.
From www.altexsoft.com
AI in Drug Discovery and Repurposing Benefits, Approaches, and Use Drug Testing Phases Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. They allow the safety and effectiveness of new drugs or treatments to be properly. Phase 1 is the first testing in humans, primarily to. Drug Testing Phases.
From www.youtube.com
GCSE Biology (Science) Drug Development YouTube Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Clinical trials and their individual phases are a very important part of clinical research. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. They allow the safety and effectiveness. Drug Testing Phases.
From medicine.st-andrews.ac.uk
Clinical Trials TB Trials University of St Andrews Drug Testing Phases Let’s take a closer look at each stage to better understand what goes into early clinical. The drug is usually approved for use in. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Find out the differences between. Clinical trials and their individual phases are a very important part of clinical. Drug Testing Phases.
From www.slideteam.net
Drug Testing Phases Ppt Powerpoint Presentation Ideas Microsoft Cpb Drug Testing Phases Find out the differences between. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Let’s take a closer look at each stage to better understand what goes into early. Drug Testing Phases.
From lustgarten.org
Clinical Trial Phases Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Let’s take a closer look. Drug Testing Phases.
From www.slidegeeks.com
Drug Testing And Clinical Trial Phases Themes PDF PowerPoint Templates Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Find out the differences between. The drug is usually approved for use in. Phase 1 is the first testing in. Drug Testing Phases.
From www.slideteam.net
Five Phases Of Drug Development Process In Pharmaceutical Company PPT Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. Let’s take a closer look at each stage to better understand what goes into early clinical. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. The drug is usually approved for use in. Clinical trials and their individual phases are. Drug Testing Phases.
From toolbox.eupati.eu
Nonclinical testing EUPATI Toolbox Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. Find out the differences between. Learn what. Drug Testing Phases.
From www.abbvieclinicaltrials.com
What Are The Phases Of Clinical Trials Clinical Trial Phases Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Find out the. Drug Testing Phases.
From www.slideteam.net
New Drug Development Process Clinical Trials Phases With Discovery Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Phase 1 is the first testing in humans, primarily to test. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Learn about the different stages of clinical testing. Drug Testing Phases.
From www.compoundchem.com
Understanding the Drug Discovery Process Compound Interest Drug Testing Phases Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. Find out the differences between. They allow the safety and effectiveness of new drugs or treatments to be. Drug Testing Phases.
From www.lancaster.ac.uk
Historical Clinical Trials Lídia André Drug Testing Phases Let’s take a closer look at each stage to better understand what goes into early clinical. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Phase 1 is the first testing in humans, primarily to test. The drug is usually approved for use in. Find out the. Drug Testing Phases.
From www.mdpi.com
IJMS Free FullText Application of Computational Biology and Drug Testing Phases They allow the safety and effectiveness of new drugs or treatments to be properly. Find out the differences between. Let’s take a closer look at each stage to better understand what goes into early clinical. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. The entire process. Drug Testing Phases.
From www.cern-foundation.org
Clinical Trial Phases CERN Foundation Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. They allow the safety and effectiveness of new drugs or treatments to be properly. Let’s take a closer look at each stage to better understand what goes into early clinical. Phase 1 is the first testing in humans,. Drug Testing Phases.
From www.researchgate.net
1 The phases of clinical trial research. Clinical trials pass through a Drug Testing Phases Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Find out the differences between. The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. Learn about the different stages of clinical testing of a new drug, from. Drug Testing Phases.
From www.slideteam.net
Clinical Trial Phases Funnel With Drug Compounds Research Design For Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. The drug is usually approved for use in. Find out the differences between. Phase 1 is the first testing. Drug Testing Phases.
From anatomisebiostats.com
Clinical Trial Phases in Drug Development Biostatistics Drug Testing Phases The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. Find out the differences between. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. They allow the safety and effectiveness of new drugs or treatments to. Drug Testing Phases.
From www.researchgate.net
Timeline showing the successive phases of training and drug testing Drug Testing Phases The drug is usually approved for use in. Let’s take a closer look at each stage to better understand what goes into early clinical. They allow the safety and effectiveness of new drugs or treatments to be properly. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Drug trials in human. Drug Testing Phases.
From www.biostock.se
Drug development The four phases BioStock Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Clinical. Drug Testing Phases.
From cancercure-d.blogspot.com
CancerCure FDA Drug Approval Process Drug Testing Phases Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Find out the differences between. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. Let’s take a closer look at each stage to better understand what goes into early. Drug Testing Phases.
From www.slideserve.com
PPT Clinical Trial Process An Overview PowerPoint Presentation ID Drug Testing Phases Phase 1 is the first testing in humans, primarily to test. Find out the differences between. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. The drug is usually approved for use in. Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least. Drug Testing Phases.
From mavink.com
Phases Of Clinical Trials Chart Drug Testing Phases Drug trials in human subjects are generally subdivided into five phases, with each phase comprised of at least one distinct clinical trial. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. The drug is usually approved for use in. Learn what phases of clinical trials are, how they are set. Drug Testing Phases.
From www.slideteam.net
Drug Testing And Clinical Trial Phases PPT Slide Drug Testing Phases Let’s take a closer look at each stage to better understand what goes into early clinical. Phase 1 is the first testing in humans, primarily to test. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. They allow the safety and effectiveness of new drugs or treatments to be properly. The. Drug Testing Phases.
From lupustrials.org
Phases of a Trial Treatment Lupus Clinical Trials Drug Testing Phases They allow the safety and effectiveness of new drugs or treatments to be properly. Phase 1 is the first testing in humans, primarily to test. The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Find out the differences between. Let’s take a closer look at each stage to better understand. Drug Testing Phases.
From www.slideteam.net
New Drug Development Process Clinical Trial Phases Funnel With Drug Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. The drug is usually approved for use in. They allow the safety and effectiveness of new drugs or treatments to be properly. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Phase. Drug Testing Phases.
From eitanmedical.com
DRUG DELIVERY DEVICES THROUGHOUT THE DRUG DEVELOPMENT CYCLE Eitan Medical Drug Testing Phases The entire process of moving a drug from design to clinical trials takes 10 to 12 years on average. Phase 1 is the first testing in humans, primarily to test. They allow the safety and effectiveness of new drugs or treatments to be properly. Clinical trials and their individual phases are a very important part of clinical research. Drug trials. Drug Testing Phases.
From www.eurofinsus.com
Bio/Pharma Product Testing Services Eurofins USA Drug Testing Phases They allow the safety and effectiveness of new drugs or treatments to be properly. Let’s take a closer look at each stage to better understand what goes into early clinical. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Learn about the different stages of clinical testing of a new drug,. Drug Testing Phases.
From www.patheon.jp
医薬品開発の5つの段階を探る Patheon pharma services Drug Testing Phases Find out the differences between. Phase 1 is the first testing in humans, primarily to test. They allow the safety and effectiveness of new drugs or treatments to be properly. The drug is usually approved for use in. Clinical trials and their individual phases are a very important part of clinical research. Drug trials in human subjects are generally subdivided. Drug Testing Phases.
From www.crbgroup.com
The basics and the process of drug approval CRB Drug Testing Phases Find out the differences between. Clinical trials and their individual phases are a very important part of clinical research. Learn what phases of clinical trials are, how they are set up, and what they aim to achieve. Learn about the different stages of clinical testing of a new drug, from phase 1 to phase 4. Drug trials in human subjects. Drug Testing Phases.