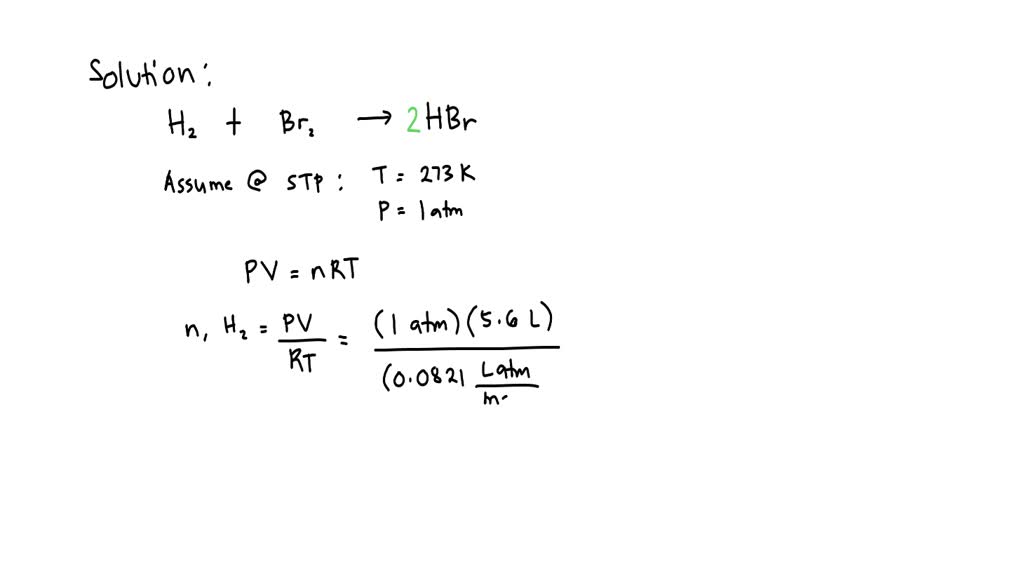Hydrogen Bromide Lithium Hydroxide Reaction . Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Libr:(1 × 6.941g/mol li) + (1 ×. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. For example, in the reaction of. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. Lioh + hbr → libr + h 2 o It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Find out what type of reaction occured. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Balance any equation or reaction using this chemical equation balancer!
from www.numerade.com
Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Lioh + hbr → libr + h 2 o Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Libr:(1 × 6.941g/mol li) + (1 ×. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! For example, in the reaction of.
SOLVED For the reaction between hydrogen and bromine which produces
Hydrogen Bromide Lithium Hydroxide Reaction (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Find out what type of reaction occured. Lioh + hbr → libr + h 2 o In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. Balance any equation or reaction using this chemical equation balancer! Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. For example, in the reaction of. Libr:(1 × 6.941g/mol li) + (1 ×. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water.
From www.numerade.com
SOLVED Write the balanced equation for the neutralization reaction of Hydrogen Bromide Lithium Hydroxide Reaction Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li]. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT Collision Theory and the Rate of Reaction PowerPoint Presentation Hydrogen Bromide Lithium Hydroxide Reaction For example, in the reaction of. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.science-revision.co.uk
Alkene addition reactions Hydrogen Bromide Lithium Hydroxide Reaction It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Balance any equation or reaction using this chemical equation balancer! (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Libr:(1 ×. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVED For the reaction of hydrogen and bromine to form hydrogen Hydrogen Bromide Lithium Hydroxide Reaction Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Libr:(1 × 6.941g/mol li) + (1 ×. It shows the reactants (substances that start a reaction) and products (substances formed. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.coursehero.com
[Solved] Quiz Exam 2 Predict the product for the following reaction Hydrogen Bromide Lithium Hydroxide Reaction In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr:(1 × 6.941g/mol li) + (1 ×. Find out what type of reaction occured. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry Hydrogen Bromide Lithium Hydroxide Reaction For example, in the reaction of. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.mdpi.com
Molecules Free FullText Tetrabutylammonium Bromide (TBAB Hydrogen Bromide Lithium Hydroxide Reaction Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Balance any equation or reaction using this chemical equation balancer! Lioh + hbr → libr + h 2 o Libr:(1 × 6.941g/mol li) + (1 ×. It shows the reactants (substances that start a reaction) and products (substances formed by the. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVED For the reaction between hydrogen and bromine which produces Hydrogen Bromide Lithium Hydroxide Reaction Lioh + hbr → libr + h 2 o For example, in the reaction of. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. Find. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT CCEA organic reaction mechanisms PowerPoint Presentation, free Hydrogen Bromide Lithium Hydroxide Reaction Find out what type of reaction occured. Lioh + hbr → libr + h 2 o Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Balance any equation or reaction using this chemical equation balancer! For example, in the reaction of. Libr:(1 ×. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT Bell Work PowerPoint Presentation, free download ID4272548 Hydrogen Bromide Lithium Hydroxide Reaction Libr:(1 × 6.941g/mol li) + (1 ×. Find out what type of reaction occured. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Balance any equation or reaction using this chemical equation balancer! In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.purechemistry.org
Of The HydrogenBromide Reaction Purechemistry Hydrogen Bromide Lithium Hydroxide Reaction (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. Balance any equation or reaction using this chemical equation balancer! It shows the reactants (substances that start a reaction) and products. Hydrogen Bromide Lithium Hydroxide Reaction.
From pubs.rsc.org
173. Reaction mechanism of the oxidation of hydrogen bromide Journal Hydrogen Bromide Lithium Hydroxide Reaction (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.youtube.com
How to Balance Li + H2 = LiH Lithium + Hydrogen gas (500700°C) YouTube Hydrogen Bromide Lithium Hydroxide Reaction Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium. Hydrogen Bromide Lithium Hydroxide Reaction.
From vpics.homes
Lithium And Water Equation Hydrogen Bromide Lithium Hydroxide Reaction Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Libr:(1 × 6.941g/mol li) + (1 ×. Lioh + hbr → libr + h 2 o Find out what type of reaction occured. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Libr +. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.chegg.com
Solved The addition of hydrogen bromide to the alkene shown Hydrogen Bromide Lithium Hydroxide Reaction Libr:(1 × 6.941g/mol li) + (1 ×. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Lioh + hbr → libr + h 2 o Li +. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVED Lithium hydroxide reacts with hydrobromic acid to produce Hydrogen Bromide Lithium Hydroxide Reaction Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. It shows the reactants (substances that start a reaction). Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Hydrogen Bromide Lithium Hydroxide Reaction Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Libr:(1 × 6.941g/mol li) + (1 ×. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Balance any equation or. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.studypool.com
SOLUTION Explaining the peroxide effect in the addition of hydrogen Hydrogen Bromide Lithium Hydroxide Reaction (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr:(1 × 6.941g/mol li) + (1 ×. For example, in the reaction of. Lioh + hbr → libr + h 2 o Lithium hydroxide and hydrobromic acid (aqueous. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.solvedlib.com
The reaction of lithium metal and water to form lithi… SolvedLib Hydrogen Bromide Lithium Hydroxide Reaction For example, in the reaction of. Libr:(1 × 6.941g/mol li) + (1 ×. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium. Hydrogen Bromide Lithium Hydroxide Reaction.
From informacionpublica.svet.gob.gt
Hydrogen Bromide Formula informacionpublica.svet.gob.gt Hydrogen Bromide Lithium Hydroxide Reaction Balance any equation or reaction using this chemical equation balancer! In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Lithium hydroxide and hydrobromic acid (aqueous. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT AQA organic reaction mechanisms PowerPoint Presentation ID159541 Hydrogen Bromide Lithium Hydroxide Reaction Lioh + hbr → libr + h 2 o Find out what type of reaction occured. For example, in the reaction of. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.coursehero.com
[Solved] What would be the products of reaction of hydrogen bromide Hydrogen Bromide Lithium Hydroxide Reaction For example, in the reaction of. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Lioh + hbr → libr + h 2 o Libr:(1 × 6.941g/mol li) + (1 ×. In equation \ (\pageindex {11}\),. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.alamy.com
Hydrogen bromide (HBr) molecule. Skeletal formula Stock Vector Image Hydrogen Bromide Lithium Hydroxide Reaction Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles of solid lithium [li] and two moles of aqueous. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Libr +. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.chegg.com
Solved Consider The Following Mechanism For The Oxidation... Hydrogen Bromide Lithium Hydroxide Reaction For example, in the reaction of. Lioh + hbr → libr + h 2 o Libr:(1 × 6.941g/mol li) + (1 ×. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Balance any equation or reaction using this chemical equation balancer! In equation \ (\pageindex {11}\), for example, the products of the reaction. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.youtube.com
Ethene + Hydrogen Bromide (Reaction and Mechanism) YouTube Hydrogen Bromide Lithium Hydroxide Reaction It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). Lioh + hbr → libr + h 2 o For example, in the reaction of. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Libr:(1 × 6.941g/mol li) + (1 ×. Find out what. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVED The reaction below shows the formation of hydrogen bromide gas Hydrogen Bromide Lithium Hydroxide Reaction Balance any equation or reaction using this chemical equation balancer! It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). For example, in the reaction of. Lioh + hbr → libr + h 2 o (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Lithium hydroxide and hydrobromic acid. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.slideserve.com
PPT Reactions of alkenes PowerPoint Presentation, free download ID Hydrogen Bromide Lithium Hydroxide Reaction Balance any equation or reaction using this chemical equation balancer! For example, in the reaction of. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an.. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVEDCalculate the reaction enthalpy for the synthesis of hydrogen Hydrogen Bromide Lithium Hydroxide Reaction Lioh + hbr → libr + h 2 o Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Balance any equation or reaction using this chemical equation balancer! For example, in the reaction of. Li + hbr = libr + h2 is a single displacement (substitution) reaction where two moles. Hydrogen Bromide Lithium Hydroxide Reaction.
From solvedlib.com
Hydrogen gas and bromine gas react to form hydrogen b… SolvedLib Hydrogen Bromide Lithium Hydroxide Reaction It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. For example, in the reaction of. Find out what type of. Hydrogen Bromide Lithium Hydroxide Reaction.
From mammothmemory.net
Lithium reacting with water gives off hydrogen gas hydroxide Hydrogen Bromide Lithium Hydroxide Reaction Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Balance. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Hydrogen Bromide Lithium Hydroxide Reaction Libr:(1 × 6.941g/mol li) + (1 ×. For example, in the reaction of. It shows the reactants (substances that start a reaction) and products (substances formed by the reaction). (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of. Hydrogen Bromide Lithium Hydroxide Reaction.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Hydrogen Bromide Lithium Hydroxide Reaction In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Libr:(1 × 6.941g/mol li) + (1 ×. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. Find out. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.chegg.com
Solved P1 The law for the hydrogenbromine reaction Hydrogen Bromide Lithium Hydroxide Reaction Libr:(1 × 6.941g/mol li) + (1 ×. Lioh + hbr → libr + h 2 o (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium ion, here an. Find out what type of reaction occured. For example, in the reaction. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Hydrogen Bromide Lithium Hydroxide Reaction Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Lithium hydroxide and hydrobromic acid (aqueous solution of hydrogen bromide) will precipitate lithium bromide in the presence of water. In equation \ (\pageindex {11}\), for example, the products of the reaction are the hydronium. Hydrogen Bromide Lithium Hydroxide Reaction.
From www.numerade.com
SOLVED Give the major organic product formed when hydrogen bromide Hydrogen Bromide Lithium Hydroxide Reaction Libr:(1 × 6.941g/mol li) + (1 ×. Libr + h2o = hbr + lioh is a double displacement (metathesis) reaction where one mole of aqueous lithium bromide [libr] and one mole of liquid. Find out what type of reaction occured. (1 ×6.941g/mol li) + (1 ×15.999g/mol o) +(1 ×1.00794g/mol h) = 23.94794 g/mol lioh. It shows the reactants (substances that. Hydrogen Bromide Lithium Hydroxide Reaction.