Titration Curve Half Equivalence Point . The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. Thus titration methods can be. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The equivalence point of a titration. It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve.
from fyoyyrbxr.blob.core.windows.net
When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. The equivalence point of a titration. Thus titration methods can be. It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like.
Equivalence Point Strong Titration at Darlene Turner blog
Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. Thus titration methods can be. Half equivalence point is exactly what it sounds like. It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch.
From fity.club
Titration Curve Titration Curve Half Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. The equivalence point of a titration. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. It is the point. Titration Curve Half Equivalence Point.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. It is the point where the volume added is half of what it will be at the equivalence point. Thus titration methods can be. The acid dissociation constant (k a) for a weak acid (ha) can be. Titration Curve Half Equivalence Point.
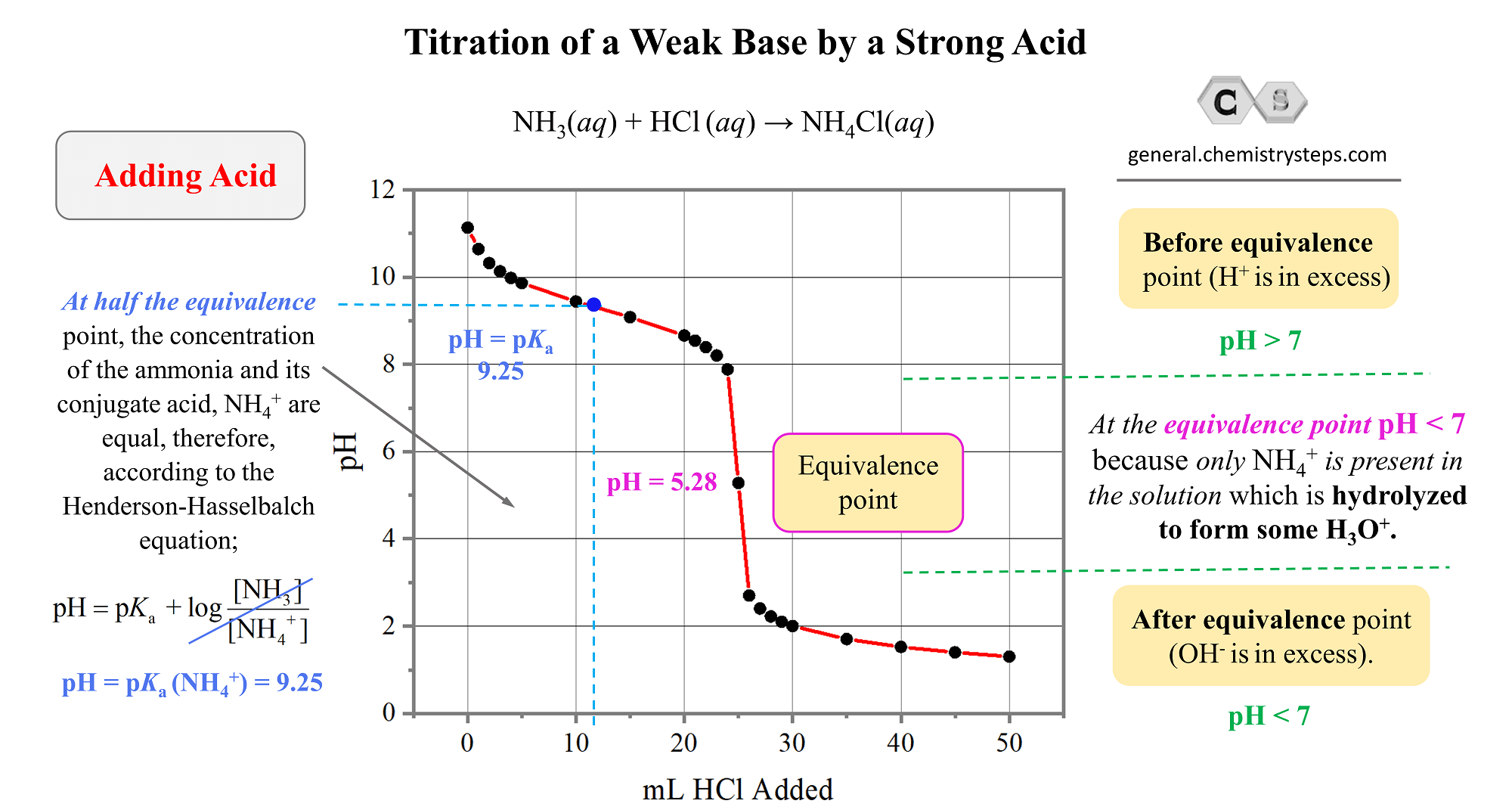
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Half Equivalence Point It is the point where the volume added is half of what it will be at the equivalence point. Thus titration methods can be. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. Half equivalence point is exactly what it sounds like. The ph at the midpoint, the point. Titration Curve Half Equivalence Point.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Titration Curve Half Equivalence Point Half equivalence point is exactly what it sounds like. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The equivalence point of a titration. It is the point where the volume added is half of what it will be at the equivalence point. When solutions of. Titration Curve Half Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Half Equivalence Point The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The equivalence point of a titration. Thus titration methods can be. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. Half equivalence point is exactly what. Titration Curve Half Equivalence Point.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Titration Curve Half Equivalence Point When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The equivalence point of a titration. It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point is where the amount of moles of acid and base are equal,. Titration Curve Half Equivalence Point.
From fyoyyrbxr.blob.core.windows.net
Equivalence Point Strong Titration at Darlene Turner blog Titration Curve Half Equivalence Point The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water.. Titration Curve Half Equivalence Point.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. It is the point where the volume added is half of what it will be at the equivalence point. Thus titration methods can be. Half equivalence point is exactly what it sounds like. The equivalence point of. Titration Curve Half Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Half Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. The equivalence point of a titration. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The equivalence. Titration Curve Half Equivalence Point.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Titration Curve Half Equivalence Point The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like. Thus titration methods. Titration Curve Half Equivalence Point.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half Titration Curve Half Equivalence Point The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. It is the point where the volume added is half of what it will be at the equivalence point. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch.. Titration Curve Half Equivalence Point.
From www.numerade.com
SOLVEDSketch the titration curve from problem 123 by calculating the Titration Curve Half Equivalence Point Thus titration methods can be. It is the point where the volume added is half of what it will be at the equivalence point. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. When solutions of some. Titration Curve Half Equivalence Point.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Half Equivalence Point It is the point where the volume added is half of what it will be at the equivalence point. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The equivalence point. Titration Curve Half Equivalence Point.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph Titration Curve Half Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point is where the amount of moles. Titration Curve Half Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Titration Curve Half Equivalence Point Thus titration methods can be. The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The ph at. Titration Curve Half Equivalence Point.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there Titration Curve Half Equivalence Point When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. Half equivalence point is exactly what it sounds like. The acid dissociation constant (k a) for a. Titration Curve Half Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The equivalence point of a titration. It is the point where the volume added is half of what it will be at the equivalence point. The ph at the midpoint, the point halfway on the titration curve. Titration Curve Half Equivalence Point.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Half Equivalence Point When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised. Titration Curve Half Equivalence Point.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point Titration Curve Half Equivalence Point Half equivalence point is exactly what it sounds like. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. Thus titration methods can be. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. Titration Curve Half Equivalence Point.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Titration Curve Half Equivalence Point When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. Thus titration methods can be. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. Half equivalence point is exactly what it sounds like. It is the. Titration Curve Half Equivalence Point.
From socratic.org
Titration curve? Please help... Socratic Titration Curve Half Equivalence Point Thus titration methods can be. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. The acid dissociation. Titration Curve Half Equivalence Point.
From fyokaiuor.blob.core.windows.net
What Is The Half Titration Point at Sarah Brandow blog Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. Thus titration methods can be. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. Half equivalence point is exactly what it sounds like.. Titration Curve Half Equivalence Point.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curve Half Equivalence Point Thus titration methods can be. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. It is the point where the volume added is half of what it will be at the equivalence point. When solutions of some polyprotic acids are titrated with strong base, not all. Titration Curve Half Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Half Equivalence Point Half equivalence point is exactly what it sounds like. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the. Titration Curve Half Equivalence Point.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Curve Half Equivalence Point Half equivalence point is exactly what it sounds like. It is the point where the volume added is half of what it will be at the equivalence point. Thus titration methods can be. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. The half equivalence point. Titration Curve Half Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration Curve Half Equivalence Point The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. The equivalence point of a titration. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The half equivalence point is the stage of the titration at which exactly half the amount. Titration Curve Half Equivalence Point.
From mungfali.com
Equivalence Points On Titration Graph Titration Curve Half Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. When solutions of some polyprotic. Titration Curve Half Equivalence Point.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The equivalence point of a titration. The acid dissociation constant (k a) for a weak acid (ha). Titration Curve Half Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Titration Curve Half Equivalence Point The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. It is the point where the volume added. Titration Curve Half Equivalence Point.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Half Equivalence Point The equivalence point of a titration. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. When solutions of some polyprotic acids are titrated with strong base, not all. Titration Curve Half Equivalence Point.
From mungfali.com
Half Equivalence Point On Titration Curve Titration Curve Half Equivalence Point The acid dissociation constant (k a) for a weak acid (ha) can be found using a titration curve. It is the point where the volume added is half of what it will be at the equivalence point. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the. Titration Curve Half Equivalence Point.
From www.youtube.com
Titration Curves Equivalence Point and Half Equivalence Point YouTube Titration Curve Half Equivalence Point It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point of a titration. When solutions of some polyprotic acids are titrated with strong base, not all of the equivalence points can be observed. The ph at the midpoint, the point halfway on the titration curve to the equivalence. Titration Curve Half Equivalence Point.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Half Equivalence Point Thus titration methods can be. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch. The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. It is the point where the volume added is. Titration Curve Half Equivalence Point.
From www.chegg.com
Solved The halfequivalence point of a titration occurs half Titration Curve Half Equivalence Point The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid or the \(pk_b\) of the weak base. Thus titration methods can be. The half equivalence point is the stage of the titration at which exactly half the amount of weak acid has been neutralised [ch.. Titration Curve Half Equivalence Point.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Titration Curve Half Equivalence Point The equivalence point is where the amount of moles of acid and base are equal, resulting a solution of only salt and water. It is the point where the volume added is half of what it will be at the equivalence point. Thus titration methods can be. When solutions of some polyprotic acids are titrated with strong base, not all. Titration Curve Half Equivalence Point.