Titration Of Hf And Naoh . You will be able to: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Relating titrations to stoichiometry and limiting reagent problems. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Calculate the ph at these volumes of. (a) locating the equivalence point volume;. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Multiply the molarity of the strong base naoh by the volume of.
from www.numerade.com
As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: (a) locating the equivalence point volume;. Relating titrations to stoichiometry and limiting reagent problems. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Multiply the molarity of the strong base naoh by the volume of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and.
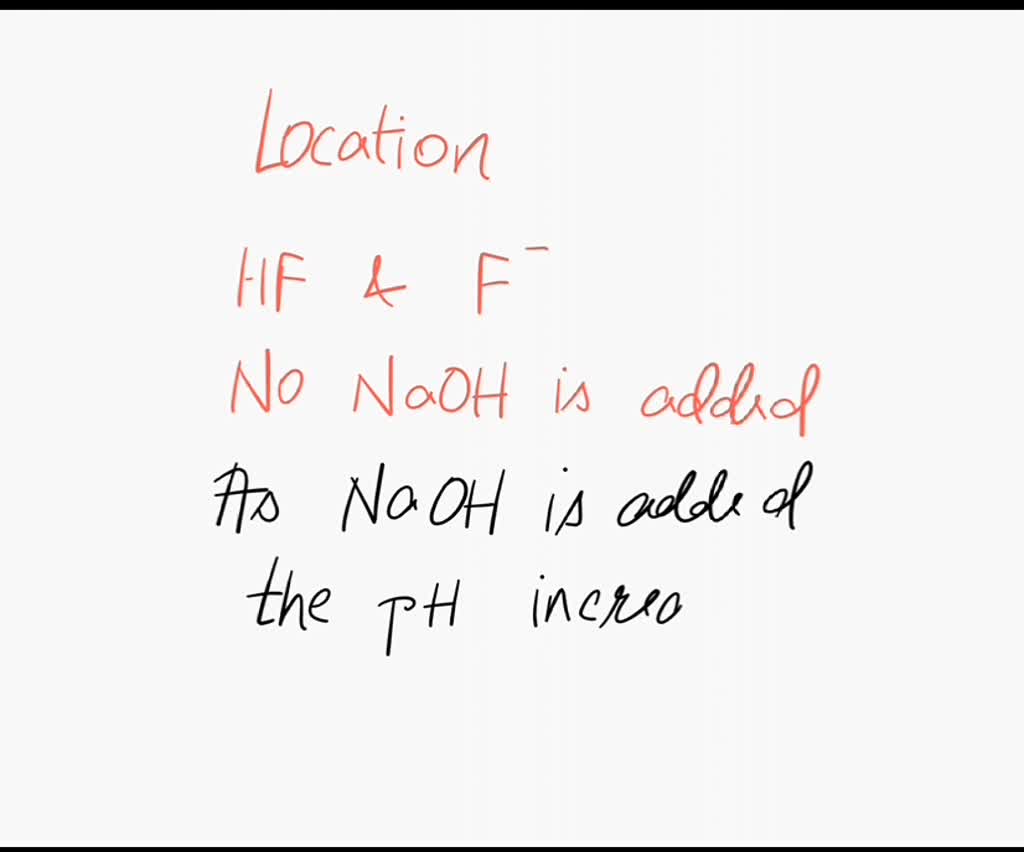
SOLVED For the titration of HF with NaOH; which location on the
Titration Of Hf And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Multiply the molarity of the strong base naoh by the volume of. (a) locating the equivalence point volume;. Calculate the ph at these volumes of. Relating titrations to stoichiometry and limiting reagent problems. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). You will be able to: Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the.
From gionfenor.blob.core.windows.net
Hcl And Naoh Titration Indicator at Lessie Breaux blog Titration Of Hf And Naoh Calculate the ph at these volumes of. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). (a) locating the equivalence. Titration Of Hf And Naoh.
From www.numerade.com
A student performed a titration of HF(aq) with NaOH(aq). The net ionic Titration Of Hf And Naoh Relating titrations to stoichiometry and limiting reagent problems. (a) locating the equivalence point volume;. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The shape of a titration curve, a. Titration Of Hf And Naoh.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Titration Of Hf And Naoh Multiply the molarity of the strong base naoh by the volume of. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. You will be able to: The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. The titration of a weak. Titration Of Hf And Naoh.
From www.chegg.com
Solved InClass Assignment 9 1. In a titration of HF (K = Titration Of Hf And Naoh Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Consider the titration. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED xamine the titration curve shown below. Which of the following Titration Of Hf And Naoh (a) locating the equivalence point volume;. Relating titrations to stoichiometry and limiting reagent problems. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED The diagrams below represent the change in the composition of a Titration Of Hf And Naoh (a) locating the equivalence point volume;. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is.. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED E3 Solution The titration curve shown below is for the Titration Of Hf And Naoh (a) locating the equivalence point volume;. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Multiply the molarity of the strong base naoh by the volume of. The titration of. Titration Of Hf And Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Titration Of Hf And Naoh (a) locating the equivalence point volume;. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Multiply the molarity of the strong base naoh by the volume of. (1) determine the hydrogen ion concentration of a weak acid via titration. Titration Of Hf And Naoh.
From www.chegg.com
Solved For the titration of HF with NaOH, which number on Titration Of Hf And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Relating titrations to. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED Write the molecular equation for a weak acid HF titration with Titration Of Hf And Naoh Calculate the ph at these volumes of. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Relating titrations to stoichiometry and limiting reagent problems. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. You will. Titration Of Hf And Naoh.
From www.numerade.com
SOLVEDConsider the titration of HF(Ka=6.7 ×10^4) with NaOH. What is Titration Of Hf And Naoh You will be able to: Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: (a) locating the equivalence point volume;. Multiply the molarity of the strong base naoh by the volume of. Calculate the ph at these volumes of. (1) determine the. Titration Of Hf And Naoh.
From chart-studio.plotly.com
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Of Hf And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions. Titration Of Hf And Naoh.
From sachiacidbase.weebly.com
Titrations Sachi's Acids and Bases Titration Of Hf And Naoh The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: You will be able to: (1) determine the hydrogen. Titration Of Hf And Naoh.
From www.numerade.com
A student performed a titration of HF(aq) with NaOH(aq). The net ionic Titration Of Hf And Naoh The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Calculate the ph at these volumes. Titration Of Hf And Naoh.
From www.chegg.com
Solved For the titration of HF with NaOH, which point on the Titration Of Hf And Naoh Calculate the ph at these volumes of. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. You will be able to: Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED Which of the titrations below best represents the graph Titration Of Hf And Naoh Relating titrations to stoichiometry and limiting reagent problems. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Multiply the molarity of the strong base naoh by the volume of. The shape of a titration curve, a plot of ph versus the amount of acid. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED Titration Lab Worksheet Prelab questions 1. 0.350 M NaOH is Titration Of Hf And Naoh (a) locating the equivalence point volume;. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. You will be able to: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The titration of a weak. Titration Of Hf And Naoh.
From courses.lumenlearning.com
15.2 AcidBase Titrations Chemistry Titration Of Hf And Naoh Relating titrations to stoichiometry and limiting reagent problems. (a) locating the equivalence point volume;. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh:. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED Determine the pH at the equivalence point for the titration of Titration Of Hf And Naoh Relating titrations to stoichiometry and limiting reagent problems. Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. You will be able to: Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: As seen in the chapter on. Titration Of Hf And Naoh.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Of Hf And Naoh (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. (a) locating the equivalence point volume;. Multiply the molarity of the strong base naoh by the volume of. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Titration Of Hf And Naoh.
From www.researchgate.net
Titration of 0.3 g etching solution (40 HNO3, 7 HF, and 30 CH3COOH Titration Of Hf And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Relating titrations to stoichiometry and limiting reagent problems. Calculate the ph at these volumes of. A titration is carried out for 25.00 ml of 0.100 m hcl. Titration Of Hf And Naoh.
From www.researchgate.net
Titration, by various bases and in various solvents, of the acids HNO 3 Titration Of Hf And Naoh You will be able to: Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. Consider the titration. Titration Of Hf And Naoh.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Of Hf And Naoh As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. You will be able to: Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. Multiply the molarity of the strong base naoh by the volume of. (1) determine the hydrogen. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED Exactly 19.36mL of 0.1481M NaOH is used to titrate a 25.00mL Titration Of Hf And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. (1) determine the hydrogen ion concentration of a weak acid via titration. Titration Of Hf And Naoh.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Of Hf And Naoh A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. The titration of a weak acid with a strong base involves the direct transfer of protons. Titration Of Hf And Naoh.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Of Hf And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). You will be able to: (1). Titration Of Hf And Naoh.
From www.numerade.com
SOLVED The diagrams below represent the change in the composition of a Titration Of Hf And Naoh Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Multiply the molarity of the strong base naoh by the volume of. Calculate the ph at these volumes of. The titration of a weak acid with a strong base involves the direct transfer. Titration Of Hf And Naoh.
From mavink.com
Titration Reaction Titration Of Hf And Naoh The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Relating titrations to stoichiometry and limiting reagent problems. A titration is carried. Titration Of Hf And Naoh.
From www.chegg.com
Solved For the titration of HF with NaOH, which location on Titration Of Hf And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. Relating titrations to stoichiometry and limiting reagent problems. You will be able to: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Calculate the ph. Titration Of Hf And Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Titration Of Hf And Naoh You will be able to: Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED For the titration of HF with NaOH, which point on the titration Titration Of Hf And Naoh As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. The titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Calculate the ph at these volumes of. Consider the titration of. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED For the titration of HF with NaOH; which location on the Titration Of Hf And Naoh Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: Relating titrations to stoichiometry and limiting reagent problems. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. As. Titration Of Hf And Naoh.
From www.numerade.com
SOLVED For the titration of HF with NaOH; which location on the Titration Of Hf And Naoh Multiply the molarity of the strong base naoh by the volume of. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in figure 14.18). Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. Calculate the ph at. Titration Of Hf And Naoh.
From www.youtube.com
How to Graph a Titration Curve (HF and NaOH) YouTube Titration Of Hf And Naoh Consider the titration of \(\ce{hcl}\) with \(\ce{naoh}\), that is, \(\ce{hcl}\) is the analyte and. The shape of a titration curve, a plot of ph versus the amount of acid or base added, provides important information about what is. Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3. Titration Of Hf And Naoh.
From ceubjufg.blob.core.windows.net
How To Do A Double Indicator Titration at Mary Frederick blog Titration Of Hf And Naoh Illustrations showing the steps used to sketch an approximate titration curve for the titration of 50.0 ml of 0.100 m ch 3 cooh with 0.200 m naoh: You will be able to: (1) determine the hydrogen ion concentration of a weak acid via titration against a strong base, (2) calculate the. Multiply the molarity of the strong base naoh by. Titration Of Hf And Naoh.