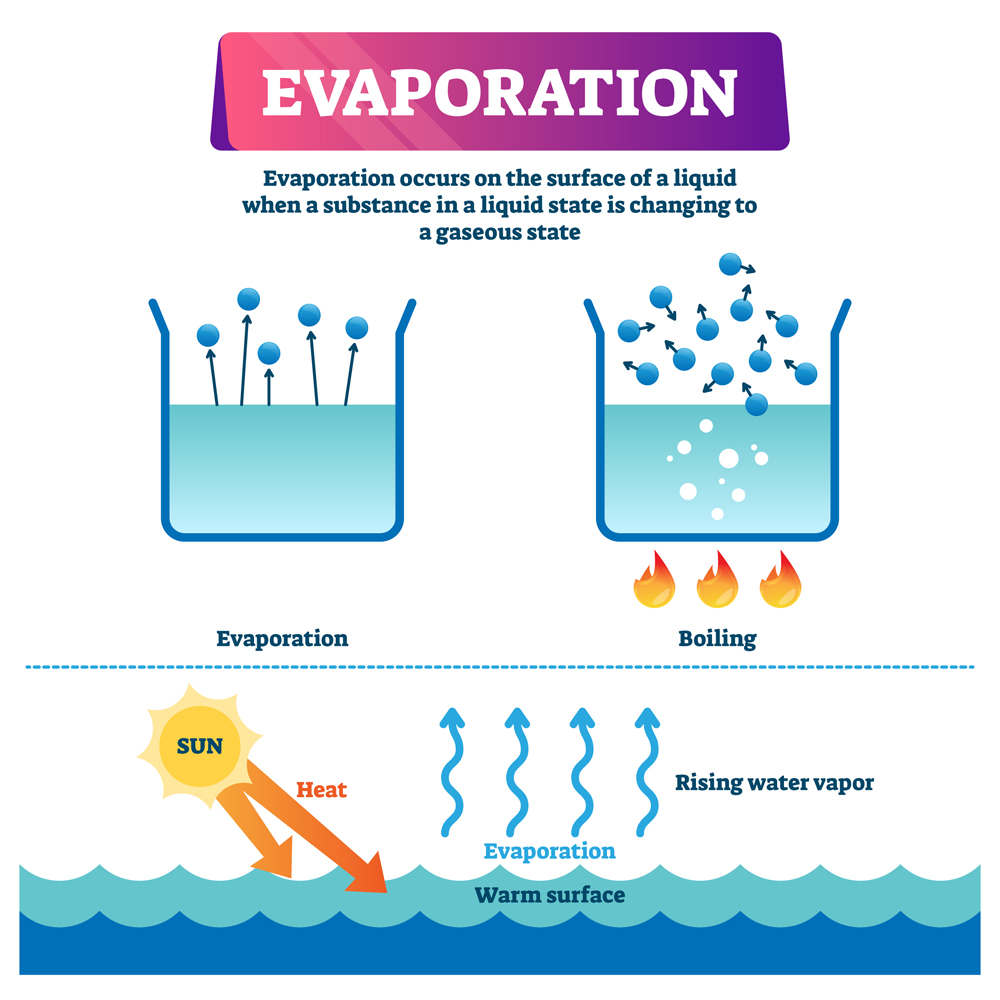
Does Water Evaporate Quickly . Around 22 pounds of water an hour (10 kg/h). How fast does pool water evaporate on a warm summer's day? Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When liquid water meets dry air, it is not in equilibrium; If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. If using hot air to evaporate. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a.
from www.scienceabc.com
When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Around 22 pounds of water an hour (10 kg/h). These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. When liquid water meets dry air, it is not in equilibrium; If using hot air to evaporate. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into.
Why Does Water Evaporate At Room Temperature?
Does Water Evaporate Quickly The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. If using hot air to evaporate. When liquid water meets dry air, it is not in equilibrium; These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. How fast does pool water evaporate on a warm summer's day? Around 22 pounds of water an hour (10 kg/h).
From www.youtube.com
How fast does water evaporate? YouTube Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. If water evaporates at room temperature because a small percentage of the molecules have enough energy to. Does Water Evaporate Quickly.
From slcc.pressbooks.pub
8.3 Controls of Weather and Climate Physical Geography and Natural Does Water Evaporate Quickly If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%).. Does Water Evaporate Quickly.
From www.animalia-life.club
Evaporates Does Water Evaporate Quickly How fast does pool water evaporate on a warm summer's day? Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. These. Does Water Evaporate Quickly.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Does Water Evaporate Quickly If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When liquid water meets dry air, it is not in equilibrium; These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. When trying to make water evaporate. Does Water Evaporate Quickly.
From giocpjsdn.blob.core.windows.net
How Fast Does Water Evaporate In A Pond at Dennis Jones blog Does Water Evaporate Quickly Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because. Does Water Evaporate Quickly.
From sciencing.com
Fast Ways to Make Water Evaporate Sciencing Does Water Evaporate Quickly If using hot air to evaporate. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When trying to make water evaporate. Does Water Evaporate Quickly.
From sciencing.com
How Fast Does Water Evaporate? Sciencing Does Water Evaporate Quickly If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative. Does Water Evaporate Quickly.
From www.youtube.com
H ow quickly does water evaporate YouTube Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. When liquid water meets dry air, it is not in equilibrium; If using hot air to evaporate. Water molecules evaporate off the surface until the amount of water in the air. Does Water Evaporate Quickly.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Quickly These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. This assumes a pool size of 30. Does Water Evaporate Quickly.
From www.youtube.com
Changing State Evaporation YouTube Does Water Evaporate Quickly If using hot air to evaporate. These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). How fast does pool water evaporate. Does Water Evaporate Quickly.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). These are relatively weak, and there are. Does Water Evaporate Quickly.
From www.pinterest.com
Liquid Nitrogen and Water In A Hot Pan Liquid nitrogen, Nitrogen, Liquid Does Water Evaporate Quickly How fast does pool water evaporate on a warm summer's day? These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. This assumes a pool size of. Does Water Evaporate Quickly.
From fyoknzxam.blob.core.windows.net
Does Kerosene Evaporate at Duane Wiggins blog Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture. Does Water Evaporate Quickly.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Around 22 pounds of water an hour (10 kg/h). This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. These are relatively weak, and. Does Water Evaporate Quickly.
From giocpjsdn.blob.core.windows.net
How Fast Does Water Evaporate In A Pond at Dennis Jones blog Does Water Evaporate Quickly If using hot air to evaporate. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. If water evaporates at room temperature because a. Does Water Evaporate Quickly.
From fyocmlcvg.blob.core.windows.net
Does Water Evaporate Out Of Pool at Ronnie Hawk blog Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. How fast does pool water evaporate on a warm summer's day? Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Water will evaporate. Does Water Evaporate Quickly.
From bigtimekitchen.com
Does Water Evaporate Faster With Or Without A Lid? Does Water Evaporate Quickly If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free. Does Water Evaporate Quickly.
From acquadom.com
Why Does My Aquarium Water Evaporate So Fast AcquaDom Does Water Evaporate Quickly When liquid water meets dry air, it is not in equilibrium; These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy. Does Water Evaporate Quickly.
From www.youtube.com
Time lapse Surface area and evaporation YouTube Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. If using hot air to evaporate. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). Water easily evaporates at its boiling point (212° f, 100° c) but evaporates. Does Water Evaporate Quickly.
From physicsexperiments.eu
Evaporation of Water and Ethanol (with Thermal Imaging Camera Does Water Evaporate Quickly When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. When liquid water meets dry air, it is not in equilibrium; These are relatively weak,. Does Water Evaporate Quickly.
From tugasnaaldin.blogspot.com
English XII Adri Fadhlansyah Aldinata XII IPA 3 Does Water Evaporate Quickly The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. When liquid water meets dry air, it is not in equilibrium; Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. These. Does Water Evaporate Quickly.
From www.futurity.org
Why belly flops end with such a painful splat Futurity Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. How fast does pool water evaporate on a warm summer's day? Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture. Does Water Evaporate Quickly.
From giocpjsdn.blob.core.windows.net
How Fast Does Water Evaporate In A Pond at Dennis Jones blog Does Water Evaporate Quickly These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Around 22 pounds of water an hour (10 kg/h). When liquid water meets dry air, it is not in equilibrium; The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the. Does Water Evaporate Quickly.
From sciencing.com
How Fast Does Water Evaporate? Sciencing Does Water Evaporate Quickly Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. How fast does pool water evaporate on a warm summer's day? Water easily evaporates. Does Water Evaporate Quickly.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). When trying to make water evaporate quickly,. Does Water Evaporate Quickly.
From infraredforhealth.com
How Long Does It Take for Isopropyl Alcohol to Evaporate? Infrared Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. How fast does pool water evaporate on a warm summer's day? When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. Water molecules evaporate off the surface. Does Water Evaporate Quickly.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Does Water Evaporate Quickly When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. How fast does pool water. Does Water Evaporate Quickly.
From musicbykatie.com
Does Adding Alcohol To Water Make It Evaporate Faster? The 15 Detailed Does Water Evaporate Quickly Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). If water evaporates at room temperature because a small percentage of the molecules have enough energy. Does Water Evaporate Quickly.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. When trying to make water evaporate quickly, it is best to spread the water over a large surface area and apply heat as evenly as possible. When liquid water meets dry. Does Water Evaporate Quickly.
From sciencing.com
Fast Ways to Make Water Evaporate Sciencing Does Water Evaporate Quickly Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. If water evaporates at room temperature because a small percentage of the. Does Water Evaporate Quickly.
From www.youtube.com
Why does water evaporate at room temperature? YouTube Does Water Evaporate Quickly Water will evaporate in an atmosphere until its partial pressure has reached the vapour pressure given for the ambient temperarture (relative humidity of 100%). How fast does pool water evaporate on a warm summer's day? These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water. Does Water Evaporate Quickly.
From www.nsta.org
Q What’s the difference between evaporation and boiling? NSTA Does Water Evaporate Quickly This assumes a pool size of 30 by 15 feet, a light breeze of 3 mph, a. These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to. Does Water Evaporate Quickly.
From fyokqptpd.blob.core.windows.net
How Quickly Does Water Evaporate at Delores Kessler blog Does Water Evaporate Quickly The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. These are relatively weak, and there are always some h 2 o molecules whizzing around with enough energy to break free of their neighbours,. If water evaporates at room temperature because a small percentage of the molecules have enough energy to. Does Water Evaporate Quickly.
From ar.inspiredpencil.com
Can Evaporated Water Does Water Evaporate Quickly The world's oceans, seas, lakes and rivers provide nearly 90 percent of the moisture in the atmosphere via evaporation. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. How fast does pool water evaporate on a warm summer's day? Water easily evaporates at its boiling point (212° f, 100° c). Does Water Evaporate Quickly.
From beezzly.com
How Long Does It Take For Water to Freeze? Beezzly Does Water Evaporate Quickly Water easily evaporates at its boiling point (212° f, 100° c) but evaporates much more slowly at its freezing point because of the heat energy required to evaporate the. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water will evaporate in an atmosphere until its partial pressure has. Does Water Evaporate Quickly.