What Metals React With Stainless Steel . Incompatible with certain types of fasteners. Chromium can shrug off oxygen without corroding. Below is a galvanic reaction chart for dissimilar metals. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. For galvanic corrosion to occur, there must be: Brass and copper are a bit in no. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. In those cases where any type of metal roof or wall cladding materials are being attached to. The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. Stainless steel is an alloy made from iron, chromium, and other metals. Transplanted plain iron or steel. Different corrosion potentials of the metals within a given system; A conductive connection between the two metals; Stainless steel has a secret weapon: The addition also pushes stainless steel higher up on the nobility chart.
from www.nuclear-power.com
Chromium can shrug off oxygen without corroding. Stainless steel has a secret weapon: Below is a galvanic reaction chart for dissimilar metals. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. For galvanic corrosion to occur, there must be: Different corrosion potentials of the metals within a given system; So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. In those cases where any type of metal roof or wall cladding materials are being attached to. Brass and copper are a bit in no. Transplanted plain iron or steel.
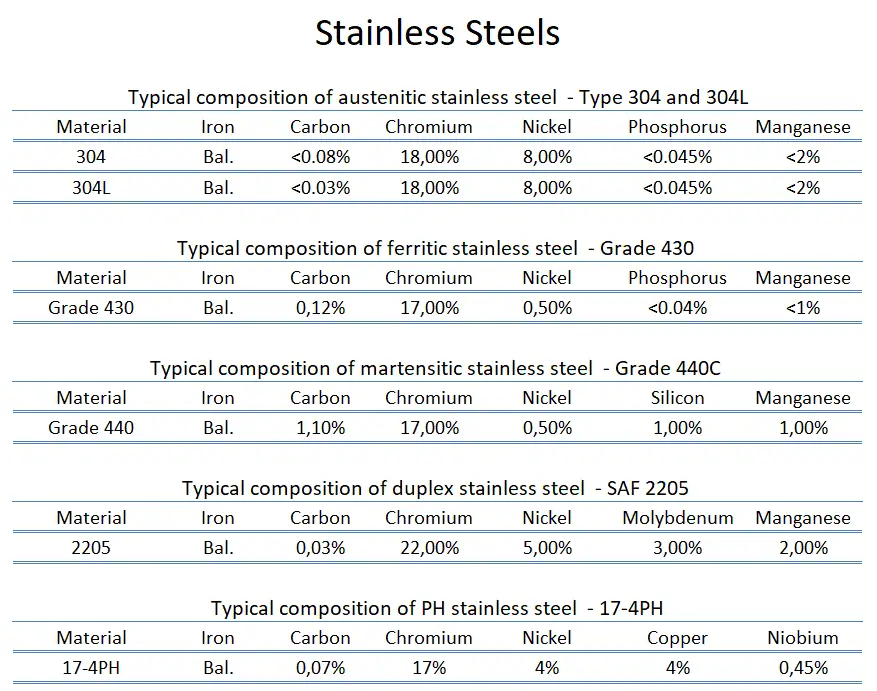
Types of Stainless Steels
What Metals React With Stainless Steel For galvanic corrosion to occur, there must be: Brass and copper are a bit in no. The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. In those cases where any type of metal roof or wall cladding materials are being attached to. A conductive connection between the two metals; Different corrosion potentials of the metals within a given system; Stainless steel is an alloy made from iron, chromium, and other metals. Incompatible with certain types of fasteners. Chromium can shrug off oxygen without corroding. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. For galvanic corrosion to occur, there must be: Stainless steel has a secret weapon: Below is a galvanic reaction chart for dissimilar metals. The addition also pushes stainless steel higher up on the nobility chart. Transplanted plain iron or steel.
From waileatowncenter.info
10 Differences Between Aluminum and Stainless Steel aluminium tube What Metals React With Stainless Steel This chart is designed to assist in broadly assessing the risk of galvanic corrosion. The addition also pushes stainless steel higher up on the nobility chart. For galvanic corrosion to occur, there must be: A conductive connection between the two metals; In those cases where any type of metal roof or wall cladding materials are being attached to. Incompatible with. What Metals React With Stainless Steel.
From www.easterneye.biz
How does stainless steel react with aluminum? EasternEye What Metals React With Stainless Steel Below is a galvanic reaction chart for dissimilar metals. A conductive connection between the two metals; This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Incompatible with certain types of fasteners. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. The. What Metals React With Stainless Steel.
From mavink.com
Chemical Resistance Chart For Metals What Metals React With Stainless Steel In those cases where any type of metal roof or wall cladding materials are being attached to. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. For galvanic corrosion to occur, there must be: A conductive connection between the two metals; Different corrosion potentials of the metals within a given system; The addition also pushes. What Metals React With Stainless Steel.
From www.rodgerindustries.com
Why we use ‘L’ Grades of Stainless Steel Knowledge Center What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. For galvanic corrosion to occur, there must be: Transplanted plain iron or steel. The addition also pushes stainless steel higher up on the nobility chart. This chart is designed to assist in broadly assessing the risk of. What Metals React With Stainless Steel.
From mavink.com
Galvanic Corrosion Aluminum Stainless Steel What Metals React With Stainless Steel In those cases where any type of metal roof or wall cladding materials are being attached to. Incompatible with certain types of fasteners. The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. The addition also pushes stainless steel higher up on the nobility chart. A conductive connection between. What Metals React With Stainless Steel.
From selftution.com
Reactivity Series of Metals and Nonmetals » Selftution What Metals React With Stainless Steel For galvanic corrosion to occur, there must be: So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. Stainless steel has a secret weapon: Transplanted plain iron or steel. Different corrosion potentials of the metals within a given system; Below is a galvanic reaction chart for dissimilar. What Metals React With Stainless Steel.
From azrust.com
Does Stainless Steel React With Aluminum? AZ Rust What Metals React With Stainless Steel The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. Different corrosion potentials of the metals within a given system; For galvanic corrosion to occur, there must be: Stainless steel is an alloy made from iron, chromium, and other metals. In those cases where any type of metal roof. What Metals React With Stainless Steel.
From recipepes.com
zinc and stainless steel reaction What Metals React With Stainless Steel Transplanted plain iron or steel. The addition also pushes stainless steel higher up on the nobility chart. Stainless steel is an alloy made from iron, chromium, and other metals. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. For galvanic corrosion to occur, there must be:. What Metals React With Stainless Steel.
From www.thefabricator.com
Understanding stainless steel formability at the atomic level What Metals React With Stainless Steel Transplanted plain iron or steel. Brass and copper are a bit in no. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. In those cases where any type of metal roof or wall cladding materials are being attached to. Different corrosion potentials of the metals within. What Metals React With Stainless Steel.
From www.onlinemathlearning.com
Reaction of Metals (examples, answers, activities, experiment, videos) What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. Chromium can shrug off oxygen without corroding. A conductive connection between the two metals; Different corrosion potentials of the metals within a given system; Stainless steel is an alloy made from iron, chromium, and other metals. The. What Metals React With Stainless Steel.
From www.madearia.com
Stainless Steel Materials 101 A Basic Knowledge Guide What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. In those cases where any type of metal roof or wall cladding materials are being attached to. A conductive connection between the two. What Metals React With Stainless Steel.
From reysrefinedradiance.wordpress.com
Stainless Steel Grades A Comprehensive Guide for Jewelry Making and What Metals React With Stainless Steel Stainless steel has a secret weapon: This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Transplanted plain iron or steel. Brass and copper are a bit in no. Different corrosion potentials of the metals within a given system; The addition also pushes stainless steel higher up on the nobility chart. Below is a galvanic reaction. What Metals React With Stainless Steel.
From www.theengineeringconcepts.com
Stainless Steel Reactor & Its Parts The Engineering Concepts What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. Brass and copper are a bit in no. Stainless steel is an alloy made from iron, chromium, and other metals. Below is a galvanic reaction chart for dissimilar metals. Chromium can shrug off oxygen without corroding. Stainless. What Metals React With Stainless Steel.
From www.iqsdirectory.com
Stainless Steel 316 What Is It? How Is It Made? Grades What Metals React With Stainless Steel Different corrosion potentials of the metals within a given system; Below is a galvanic reaction chart for dissimilar metals. A conductive connection between the two metals; The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. Brass and copper are a bit in no. So when stainless steel and. What Metals React With Stainless Steel.
From www.ulbrich.com
Saltwater Corrosion with Stainless Steel Ulbrich What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. Stainless steel has a secret weapon: The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. For galvanic corrosion to occur, there must be: Different corrosion. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Stainless Steel React with Aluminum? Exploring the Interaction What Metals React With Stainless Steel A conductive connection between the two metals; For galvanic corrosion to occur, there must be: The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. Transplanted plain iron or steel. Brass and copper are a bit in no. So when stainless steel and carbon steel are connected, and an. What Metals React With Stainless Steel.
From recipepes.com
zinc and stainless steel reaction What Metals React With Stainless Steel Transplanted plain iron or steel. Below is a galvanic reaction chart for dissimilar metals. A conductive connection between the two metals; Stainless steel is an alloy made from iron, chromium, and other metals. Stainless steel has a secret weapon: Brass and copper are a bit in no. Chromium can shrug off oxygen without corroding. Incompatible with certain types of fasteners.. What Metals React With Stainless Steel.
From www.pinterest.co.uk
MetalReactivitiesSeries Fe iron, Science blog, Metal What Metals React With Stainless Steel Below is a galvanic reaction chart for dissimilar metals. In those cases where any type of metal roof or wall cladding materials are being attached to. A conductive connection between the two metals; The addition also pushes stainless steel higher up on the nobility chart. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Stainless. What Metals React With Stainless Steel.
From blog.inoxmare.com
Corrosion and Stainless Steel How Trigger and Prevent it? What Metals React With Stainless Steel In those cases where any type of metal roof or wall cladding materials are being attached to. Transplanted plain iron or steel. Stainless steel has a secret weapon: A conductive connection between the two metals; So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. The addition. What Metals React With Stainless Steel.
From www.reddit.com
Stainless steel + Sulfuric acid ==> Beautiful crystals. Stainless steel What Metals React With Stainless Steel For galvanic corrosion to occur, there must be: Below is a galvanic reaction chart for dissimilar metals. In those cases where any type of metal roof or wall cladding materials are being attached to. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Incompatible with certain types of fasteners. Stainless steel has a secret weapon:. What Metals React With Stainless Steel.
From galvanizeit.org
Stainless Steel in Contact with… American Galvanizers Association What Metals React With Stainless Steel For galvanic corrosion to occur, there must be: A conductive connection between the two metals; Below is a galvanic reaction chart for dissimilar metals. Brass and copper are a bit in no. Incompatible with certain types of fasteners. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look What Metals React With Stainless Steel Below is a galvanic reaction chart for dissimilar metals. The addition also pushes stainless steel higher up on the nobility chart. For galvanic corrosion to occur, there must be: Incompatible with certain types of fasteners. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Transplanted plain iron or steel. Stainless steel is an alloy made. What Metals React With Stainless Steel.
From blog.thepipingmart.com
Titanium vs. Stainless Steel Corrosion Resistance What Metals React With Stainless Steel Stainless steel is an alloy made from iron, chromium, and other metals. Incompatible with certain types of fasteners. For galvanic corrosion to occur, there must be: Stainless steel has a secret weapon: Below is a galvanic reaction chart for dissimilar metals. The addition also pushes stainless steel higher up on the nobility chart. Brass and copper are a bit in. What Metals React With Stainless Steel.
From www.nuclear-power.com
Types of Stainless Steels What Metals React With Stainless Steel The addition also pushes stainless steel higher up on the nobility chart. Brass and copper are a bit in no. Incompatible with certain types of fasteners. Stainless steel is an alloy made from iron, chromium, and other metals. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. So when stainless steel and carbon steel are. What Metals React With Stainless Steel.
From pediaa.com
Difference Between Steel and Stainless Steel Definition, Composition What Metals React With Stainless Steel Stainless steel has a secret weapon: Incompatible with certain types of fasteners. Below is a galvanic reaction chart for dissimilar metals. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Chromium can. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Stainless Steel React with Aluminum? Exploring the Interaction What Metals React With Stainless Steel In those cases where any type of metal roof or wall cladding materials are being attached to. Transplanted plain iron or steel. Below is a galvanic reaction chart for dissimilar metals. Chromium can shrug off oxygen without corroding. Stainless steel is an alloy made from iron, chromium, and other metals. Brass and copper are a bit in no. Incompatible with. What Metals React With Stainless Steel.
From blog.thepipingmart.com
Brass vs. Stainless Steel Hardness and Strength What Metals React With Stainless Steel For galvanic corrosion to occur, there must be: Incompatible with certain types of fasteners. Transplanted plain iron or steel. The addition also pushes stainless steel higher up on the nobility chart. Brass and copper are a bit in no. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Different corrosion potentials of the metals within. What Metals React With Stainless Steel.
From blog.thepipingmart.com
Silver Steel vs Stainless Steel What's the Difference? What Metals React With Stainless Steel Different corrosion potentials of the metals within a given system; So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. Chromium can shrug off oxygen without corroding. Stainless steel is an alloy made. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look What Metals React With Stainless Steel Chromium can shrug off oxygen without corroding. Transplanted plain iron or steel. Stainless steel is an alloy made from iron, chromium, and other metals. Stainless steel has a secret weapon: A conductive connection between the two metals; Incompatible with certain types of fasteners. This chart is designed to assist in broadly assessing the risk of galvanic corrosion. So when stainless. What Metals React With Stainless Steel.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts What Metals React With Stainless Steel A conductive connection between the two metals; Different corrosion potentials of the metals within a given system; For galvanic corrosion to occur, there must be: So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. This chart is designed to assist in broadly assessing the risk of. What Metals React With Stainless Steel.
From blog.thepipingmart.com
How to Tarnish Stainless Steel? What Metals React With Stainless Steel A conductive connection between the two metals; The addition also pushes stainless steel higher up on the nobility chart. For galvanic corrosion to occur, there must be: Transplanted plain iron or steel. Different corrosion potentials of the metals within a given system; Chromium can shrug off oxygen without corroding. So when stainless steel and carbon steel are connected, and an. What Metals React With Stainless Steel.
From recipepes.com
zinc and stainless steel reaction What Metals React With Stainless Steel Below is a galvanic reaction chart for dissimilar metals. For galvanic corrosion to occur, there must be: The obvious point is that when using dissimilar metals in a fabrication project, precautions must be taken to prevent galvanic reaction. So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look What Metals React With Stainless Steel Brass and copper are a bit in no. The addition also pushes stainless steel higher up on the nobility chart. Incompatible with certain types of fasteners. Transplanted plain iron or steel. A conductive connection between the two metals; Different corrosion potentials of the metals within a given system; Chromium can shrug off oxygen without corroding. Stainless steel has a secret. What Metals React With Stainless Steel.
From blog.thepipingmart.com
Aluminium Alloy vs Stainless Steel What's the Difference What Metals React With Stainless Steel So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. A conductive connection between the two metals; This chart is designed to assist in broadly assessing the risk of galvanic corrosion. The obvious point is that when using dissimilar metals in a fabrication project, precautions must be. What Metals React With Stainless Steel.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look What Metals React With Stainless Steel Stainless steel has a secret weapon: A conductive connection between the two metals; So when stainless steel and carbon steel are connected, and an electrolyte such as moisture is introduced, stainless steel absorbs carbon steel’s electrons. Stainless steel is an alloy made from iron, chromium, and other metals. Different corrosion potentials of the metals within a given system; The addition. What Metals React With Stainless Steel.