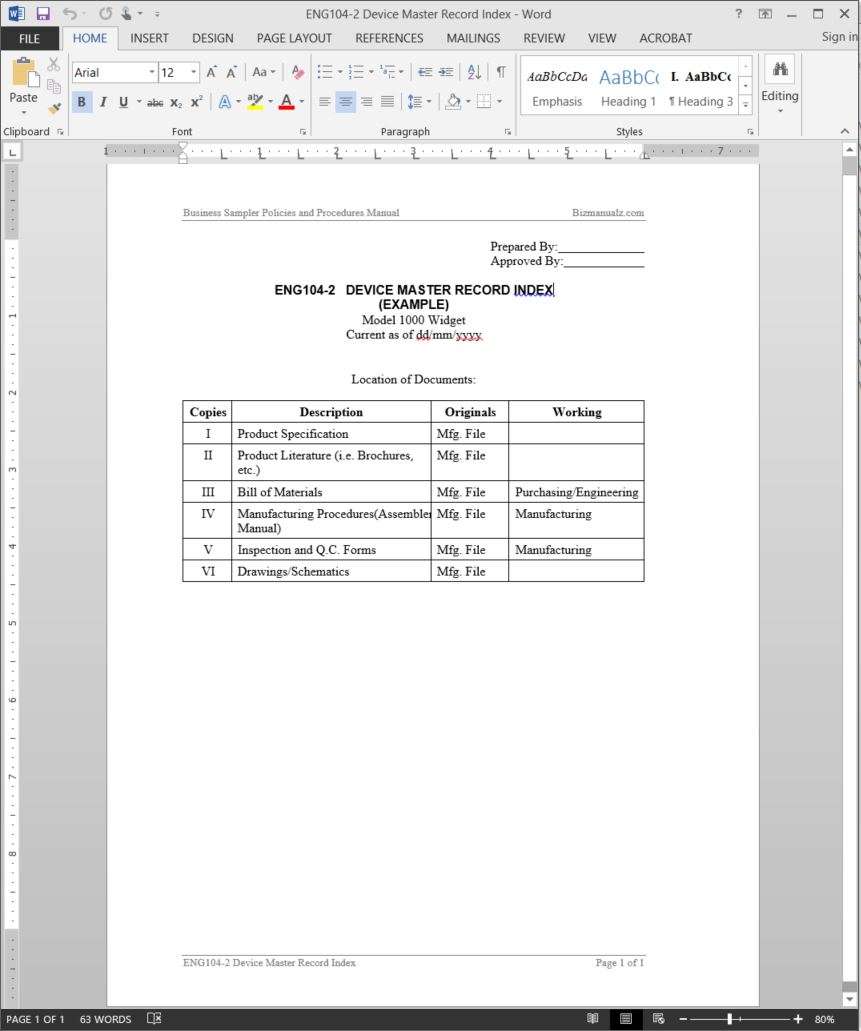
Device History Record File . — a design history file (dhf) shows the design history of a medical device. It is used to provide evidence that all the design control procedures were. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. In its essence, a dhr is a. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. What is the device history record (dhr)? — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”).
from old.sermitsiaq.ag
In its essence, a dhr is a. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” It is used to provide evidence that all the design control procedures were. What is the device history record (dhr)? the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. — a design history file (dhf) shows the design history of a medical device.
Device Master Record Template
Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. — a design history file (dhf) shows the design history of a medical device. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” In its essence, a dhr is a. — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. What is the device history record (dhr)? — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. It is used to provide evidence that all the design control procedures were.
From www.youtube.com
Design History File DHF, Device Master Record DMR, Device History Record DHR and Technical File Device History Record File Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” It is used to provide evidence that all the design control procedures were. — the fda requires a design history file dhf in. Device History Record File.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR) What's Device History Record File the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. It is used to provide evidence that all the design control procedures were. — the. Device History Record File.
From www.youtube.com
Design History File (DHF), the Device Master Record (DMR) and the Device History Record (DHR Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. — a design history file (dhf) shows the design history of a medical device. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device,. Device History Record File.
From horusb.weebly.com
Design master record design history file horusb Device History Record File What is the device history record (dhr)? — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. In its essence, a dhr is a. — a. Device History Record File.
From sterlingmedicaldevices.com
Design History Files Everything You Should Know Device History Record File What is the device history record (dhr)? the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. It is used to provide evidence that all the design control procedures were. In its essence, a dhr is a. Cfr 820.184 requires manufacturers to “establish and. Device History Record File.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” the. Device History Record File.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs. Device History Record File.
From www.youtube.com
Device History Record HD YouTube Device History Record File In its essence, a dhr is a. the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. It is used to provide evidence that all the design control procedures were. — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step.. Device History Record File.
From www.youtube.com
Design History File, Device History Record, Device Master Record and Impact of Change Controls Device History Record File the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master. Device History Record File.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR Device History Record File It is used to provide evidence that all the design control procedures were. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” the design history file (dhf) and device master record (dmr). Device History Record File.
From old.sermitsiaq.ag
Device History Record Template Device History Record File It is used to provide evidence that all the design control procedures were. — a design history file (dhf) shows the design history of a medical device. the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. Cfr 820.184 requires manufacturers to “establish and maintain procedures to. Device History Record File.
From www.qualitymeddev.com
Device History Record (DHR) An Overview QualityMedDev Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). In its essence, a dhr is a. — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. It is used to provide evidence that all the design control procedures were. . Device History Record File.
From www.slideserve.com
PPT Design documentation PowerPoint Presentation ID1625484 Device History Record File — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. the design history file (dhf) and device master record (dmr) are like a medical device. Device History Record File.
From blog.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR Device History Record File In its essence, a dhr is a. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — a design history file (dhf) shows the design history of a medical device. What is the device history record (dhr)? It is used to provide. Device History Record File.
From www.instantgmp.com
Device History Record Device History Record File Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. In its essence, a dhr is. Device History Record File.
From www.aplyon.com
Device History Record Procedure Device History Record File — a design history file (dhf) shows the design history of a medical device. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. In its essence, a dhr is a. the design history file (dhf) and device master record (dmr) are. Device History Record File.
From www.instantgmp.com
Device History Record Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” What is. Device History Record File.
From templates.rjuuc.edu.np
Medical Device Design History File Template Device History Record File — a design history file (dhf) shows the design history of a medical device. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality. Device History Record File.
From www.vrogue.co
Device Master Records Design History Files Gmp Docs vrogue.co Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — a device history record encapsulates the manufacturing narrative of a medical. Device History Record File.
From www.youtube.com
Device Master Record & Device History Record A Regulatory YouTube Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). It is used to provide evidence that all the design control procedures were. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device. Device History Record File.
From www.instantgmp.com
Device History Record Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. — a device history record encapsulates the manufacturing narrative of a medical. Device History Record File.
From templates.rjuuc.edu.np
Device History Record Template Device History Record File — a design history file (dhf) shows the design history of a medical device. In its essence, a dhr is a. the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. It is used to provide evidence that all the design control procedures were. — the. Device History Record File.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR) What's Device History Record File What is the device history record (dhr)? Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” the design history file (dhf) and device master record (dmr) are like a medical device recipe. Device History Record File.
From studylib.net
Device Master Record Device History Record File What is the device history record (dhr)? — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). — a design history file (dhf) shows the design history of a medical device. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot,. Device History Record File.
From www.youtube.com
Design History File (DHF), the Device Master Record (DMR) and the Device History Record (DHR Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). It is used to provide evidence that all the design control procedures were. — a design history file (dhf) shows the design history of a medical device. the device master record (dmr) can be thought of as. Device History Record File.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR) What's Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs. Device History Record File.
From old.sermitsiaq.ag
Device Master Record Template Device History Record File the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. It is used to provide evidence that all the design control procedures were. What is the device history record (dhr)? — the fda requires a design history file dhf in 21 cfr part. Device History Record File.
From www.arenasolutions.com
Device History Record (DHR) Definition Arena Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” —. Device History Record File.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device History Record File Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. It is used to provide evidence. Device History Record File.
From www.presentationeze.com
Device History Record (DHR). Simple explanation of US FDA requirementsPresentationEZE Device History Record File In its essence, a dhr is a. — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). What is the device history record (dhr)? the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. — a. Device History Record File.
From www.scilife.io
What is Device History Record (DHR)? Complete definition Scilife Device History Record File — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). In its essence, a dhr is a. It is used to provide evidence that all the design control procedures were. — a design history file (dhf) shows the design history of a medical device. the device master. Device History Record File.
From www.slideshare.net
Batch Production and Device History Record Quality Review for Complia… Device History Record File Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” In its essence, a dhr is a. — a design history file (dhf) shows the design history of a medical device. What is. Device History Record File.
From www.aplyon.com
Device History Record Quality Systems Device History Record File the design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design. In its essence, a dhr is a. What is the device history. Device History Record File.
From docs.oracle.com
Oracle Manufacturing Implementing Oracle ERecords in Discrete Manufacturing Guide Device History Record File Cfr 820.184 requires manufacturers to “establish and maintain procedures to ensure that dhrs for each batch, lot, or unit are maintained to demonstrate that the device is manufactured in accordance with the device master record (dmr).” In its essence, a dhr is a. the design history file (dhf) and device master record (dmr) are like a medical device recipe. Device History Record File.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR) What's Device History Record File It is used to provide evidence that all the design control procedures were. — a device history record encapsulates the manufacturing narrative of a medical device, archiving every critical step. In its essence, a dhr is a. — the fda requires a design history file dhf in 21 cfr part 820 (these are the “quality system regulations”). . Device History Record File.