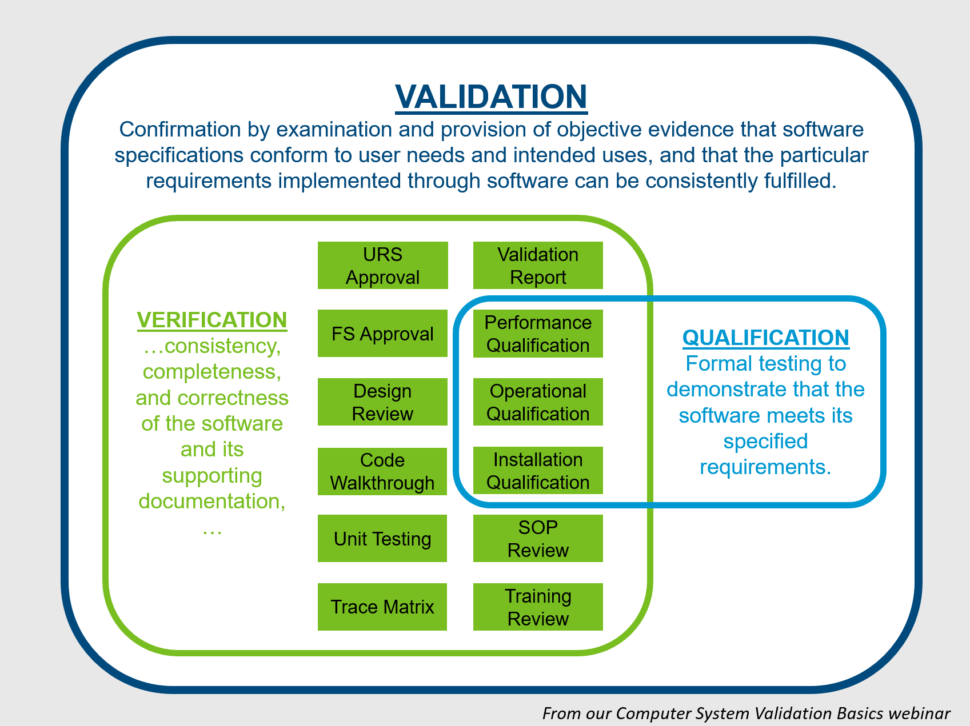
Test Method Validation Steps . — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. how does your laboratory verify or establish reference intervals? test method validation is the documented process of ensuring a test method is suitable for its intended use. Requirements to perform a validation or verification study. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. How does your laboratory validate clinical claims made. — the test method validation (tmv) process usually starts with determining which test methods on a.
from validationcenter.com
Requirements to perform a validation or verification study. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. how does your laboratory verify or establish reference intervals? — the test method validation (tmv) process usually starts with determining which test methods on a. test method validation is the documented process of ensuring a test method is suitable for its intended use. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. How does your laboratory validate clinical claims made. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision.
What is Computer System Validation and How Do You Do It?
Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the test method validation (tmv) process usually starts with determining which test methods on a. How does your laboratory validate clinical claims made. test method validation is the documented process of ensuring a test method is suitable for its intended use.
From www.lambdatest.com
Test Verification vs Validation in site Testing Test Method Validation Steps Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the test method validation (tmv) process usually starts with determining which test methods on a. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. —. Test Method Validation Steps.
From www.semanticscholar.org
Figure 2 from Stepbystep analytical methods validation and protocol in the quality system Test Method Validation Steps — the test method validation (tmv) process usually starts with determining which test methods on a. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. How does your laboratory validate clinical claims made. how does your laboratory verify or establish reference intervals? —. Test Method Validation Steps.
From www.xenonstack.com
Data Validation Testing Tools and Techniques Complete Guide Test Method Validation Steps How does your laboratory validate clinical claims made. — the test method validation (tmv) process usually starts with determining which test methods on a. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. Requirements to perform a validation or verification study. test method validation is the documented process of ensuring. Test Method Validation Steps.
From www.gmpsop.com
All infographic embed Codes from Test Method Validation Steps test method validation is the documented process of ensuring a test method is suitable for its intended use. how does your laboratory verify or establish reference intervals? — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the test method validation (tmv) process usually starts with determining which test. Test Method Validation Steps.
From exofltpju.blob.core.windows.net
What Is Test Method Validation at Karen Coulson blog Test Method Validation Steps — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. How does your laboratory validate clinical. Test Method Validation Steps.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Test Method Validation Steps how does your laboratory verify or establish reference intervals? — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the test method validation (tmv) process usually starts with determining which test. Test Method Validation Steps.
From mavink.com
Validation Process Flow Chart Test Method Validation Steps How does your laboratory validate clinical claims made. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the test method validation (tmv) process usually starts with determining which test methods on. Test Method Validation Steps.
From validationcenter.com
What is Computer System Validation and How Do You Do It? Test Method Validation Steps test method validation is the documented process of ensuring a test method is suitable for its intended use. How does your laboratory validate clinical claims made. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. — the test method validation (tmv) process usually starts with determining which test methods. Test Method Validation Steps.
From www.youtube.com
Types of Test Method Validation Medical Device (Full Online Course with Certification) YouTube Test Method Validation Steps How does your laboratory validate clinical claims made. how does your laboratory verify or establish reference intervals? test method validation is the documented process of ensuring a test method is suitable for its intended use. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation is the process. Test Method Validation Steps.
From mavink.com
Validation Process Flow Chart Test Method Validation Steps Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. —. Test Method Validation Steps.
From www.xenonstack.com
Machine learning Model Validation Testing A Quick Guide Test Method Validation Steps method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. How does your laboratory validate clinical claims made. Requirements to perform a validation or verification study. — the test method validation (tmv) process usually starts with determining which test methods on a. — the ich,. Test Method Validation Steps.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation ID8861574 Test Method Validation Steps — the test method validation (tmv) process usually starts with determining which test methods on a. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. Requirements to perform a validation or verification. Test Method Validation Steps.
From www.softwaretestinghelp.com
Validation Testing Ultimate Guide Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. — the test method validation (tmv). Test Method Validation Steps.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Presentation ID6596159 Test Method Validation Steps test method validation is the documented process of ensuring a test method is suitable for its intended use. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the.. Test Method Validation Steps.
From kvalito.ch
How is a system validated? Kvalito Test Method Validation Steps Requirements to perform a validation or verification study. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the ich, fda, and usp define the test procedure parameters to validate as encompassing. Test Method Validation Steps.
From www.youtube.com
Analytical Method Development & Validation YouTube Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. Requirements to perform a validation or. Test Method Validation Steps.
From www.labcompare.com
Guide to Method Validation of Test Procedures Test Method Validation Steps how does your laboratory verify or establish reference intervals? Requirements to perform a validation or verification study. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. test method validation is the documented process of ensuring a test method is suitable for its intended use.. Test Method Validation Steps.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online presentation Test Method Validation Steps method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. test method validation is the documented process of ensuring a test method is suitable for its intended use. Requirements to perform a validation or verification study. — the test method validation (tmv) process usually starts. Test Method Validation Steps.
From www.openvc.app
How to validate your startup idea 6 methods explained Test Method Validation Steps — the test method validation (tmv) process usually starts with determining which test methods on a. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. test method validation is the documented process of ensuring a test method is suitable for its intended use. . Test Method Validation Steps.
From www.europeanpharmaceuticalreview.com
Test method validation for cleaning validation samples Test Method Validation Steps test method validation is the documented process of ensuring a test method is suitable for its intended use. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. method validation is the process. Test Method Validation Steps.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation ID8861574 Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the test method validation (tmv) process usually starts with determining which test methods on a. Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the ich, fda, and usp define. Test Method Validation Steps.
From vitalflux.com
Hypothesis Testing Steps & Examples Analytics Yogi Test Method Validation Steps Requirements to perform a validation or verification study. how does your laboratory verify or establish reference intervals? — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. . Test Method Validation Steps.
From www.starteer.com
How to validate your startup idea in 6 easy steps Starteer Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. Requirements to perform a validation or verification study. method validation is the process by which it is established, through laboratory studies, that the performance. Test Method Validation Steps.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the test method validation (tmv) process usually starts with determining which test methods on a. — the ich, fda, and usp. Test Method Validation Steps.
From www.dober.com
Cleaning Validation Support For Critical Cleaners Test Method Validation Steps how does your laboratory verify or establish reference intervals? How does your laboratory validate clinical claims made. test method validation is the documented process of ensuring a test method is suitable for its intended use. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the.. Test Method Validation Steps.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Test Method Validation Steps how does your laboratory verify or establish reference intervals? How does your laboratory validate clinical claims made. Requirements to perform a validation or verification study. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. — the ich, fda, and usp define the test procedure. Test Method Validation Steps.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Test Method Validation Steps how does your laboratory verify or establish reference intervals? Requirements to perform a validation or verification study. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. How does your laboratory validate clinical claims made. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy,. Test Method Validation Steps.
From www.chromatographyonline.com
Validation of StabilityIndicating HPLC Methods for Pharmaceuticals Overview, Methodologies Test Method Validation Steps How does your laboratory validate clinical claims made. test method validation is the documented process of ensuring a test method is suitable for its intended use. Requirements to perform a validation or verification study. — the test method validation (tmv) process usually starts with determining which test methods on a. how does your laboratory verify or establish. Test Method Validation Steps.
From www.geeksforgeeks.org
Software Engineering Verification and Validation Test Method Validation Steps how does your laboratory verify or establish reference intervals? test method validation is the documented process of ensuring a test method is suitable for its intended use. — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. — the ich, fda, and usp define the test procedure parameters to validate. Test Method Validation Steps.
From www.template.net
Validation Report Templates 9+ Free Word, PDF Format Download Test Method Validation Steps — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. how does your laboratory verify or establish reference intervals? method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. How does your laboratory validate clinical claims made. Requirements. Test Method Validation Steps.
From www.technolush.com
Verification & Validation Model TechnoLush Test Method Validation Steps How does your laboratory validate clinical claims made. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the ich, fda, and usp define the test procedure parameters to validate as encompassing accuracy, precision. Requirements to perform a validation or verification study. method validation is the process by. Test Method Validation Steps.
From dxobsupta.blob.core.windows.net
Test Method Validation Pdf at Loretta Molina blog Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. Requirements to perform a validation or verification study. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the ich, fda, and usp define the test procedure parameters to validate as encompassing. Test Method Validation Steps.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online presentation Test Method Validation Steps — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. method validation is the process by which it is established, through laboratory studies, that the performance characteristics of the method meet the. how does your laboratory verify or establish reference intervals? — the test method validation (tmv) process usually starts. Test Method Validation Steps.
From www.youtube.com
Method Validation inar YouTube Test Method Validation Steps how does your laboratory verify or establish reference intervals? — the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. test method validation is the documented process of ensuring a test method is suitable for its intended use. — the test method validation (tmv) process usually starts with determining which test. Test Method Validation Steps.