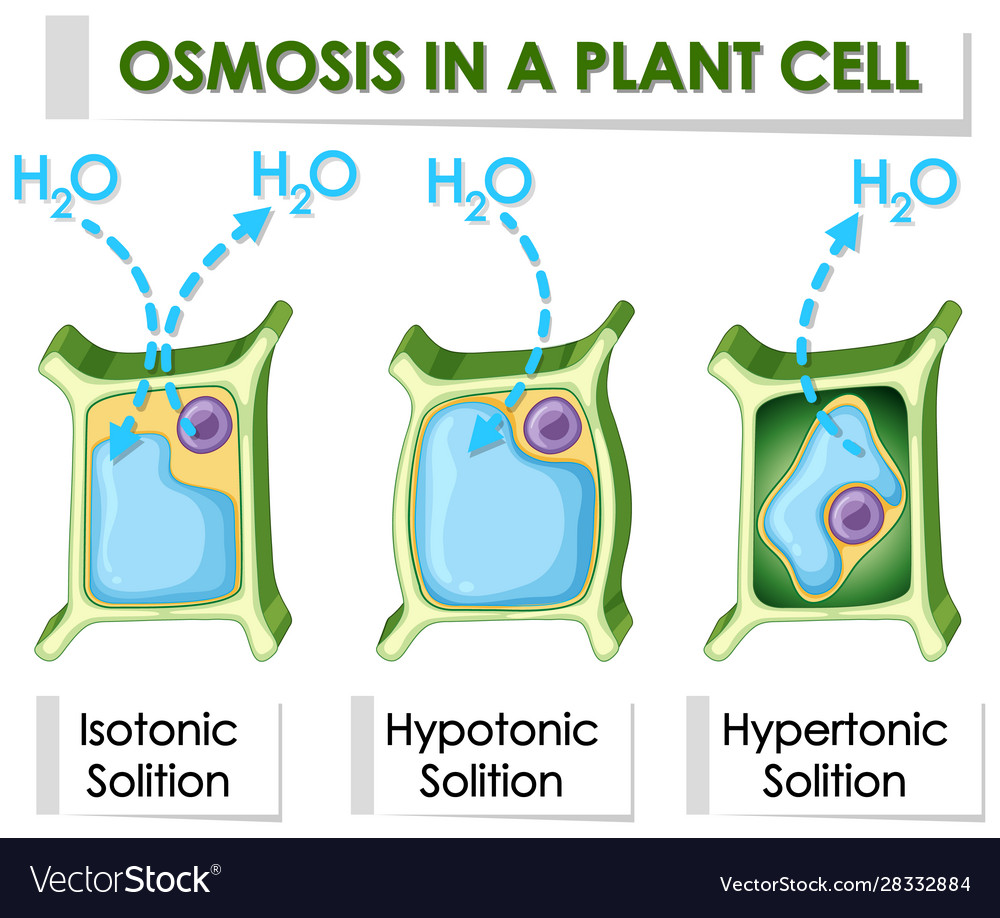
How Does Distilled Water Affect Osmosis . Water moves into and out of cells by osmosis. the osmotic pressure driving water across an impermeable barrier increases with the difference in solute concentrations on either side of the barrier. all cells are enclosed by a cell membrane, which is selectively permeable. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. The diffusion of water molecules across a semipermeable membrane. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. Molecules can move into or out of cells by diffusion. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. osmosis is a specific type of diffusion:
from www.vectorstock.com
Water moves into and out of cells by osmosis. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. The diffusion of water molecules across a semipermeable membrane. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. all cells are enclosed by a cell membrane, which is selectively permeable. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. Molecules can move into or out of cells by diffusion. osmosis is a specific type of diffusion: this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria.
Diagram showing osmosis in plant cell Royalty Free Vector
How Does Distilled Water Affect Osmosis osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. osmosis is a specific type of diffusion: all cells are enclosed by a cell membrane, which is selectively permeable. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. the osmotic pressure driving water across an impermeable barrier increases with the difference in solute concentrations on either side of the barrier. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. Water moves into and out of cells by osmosis. The diffusion of water molecules across a semipermeable membrane. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. Molecules can move into or out of cells by diffusion. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until.
From www.watersystemsguide.com
Distilled Vs Reverse Osmosis Water Water Systems Guide How Does Distilled Water Affect Osmosis osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. all cells are enclosed by a cell membrane, which is selectively permeable. The diffusion of water. How Does Distilled Water Affect Osmosis.
From www.vectorstock.com
Diagram showing osmosis in plant cell Royalty Free Vector How Does Distilled Water Affect Osmosis this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. the osmotic pressure driving water across an impermeable barrier increases with the difference in solute concentrations on either side of the barrier. the present paper explores the effect of concentration and temperature on water and salt. How Does Distilled Water Affect Osmosis.
From waterfilterguru.com
Reverse Osmosis vs Distilled Water (The 2024 Definitive Comparison) How Does Distilled Water Affect Osmosis In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. osmosis is a specific type of diffusion: all cells are enclosed by a cell membrane, which is selectively permeable. The diffusion of water molecules across a semipermeable membrane. this is similar to distilled water, but the. How Does Distilled Water Affect Osmosis.
From www.sliderbase.com
Osmoregulation and Excretion Presentation Biology How Does Distilled Water Affect Osmosis In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. all cells are enclosed by a cell membrane, which is selectively permeable. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. osmosis is the diffusion of water molecules. How Does Distilled Water Affect Osmosis.
From www.slideshare.net
Osmosis, diffusion, active transport How Does Distilled Water Affect Osmosis If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. The diffusion of water molecules across a semipermeable membrane. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. osmosis is the diffusion of water molecules across a. How Does Distilled Water Affect Osmosis.
From www.edplace.com
Understand Osmosis Worksheet EdPlace How Does Distilled Water Affect Osmosis the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. the combination of membrane permeability, driving force, and the density and identity of active transport. How Does Distilled Water Affect Osmosis.
From biologicalmembranetransport.weebly.com
Osmosis BioEdu How Does Distilled Water Affect Osmosis all cells are enclosed by a cell membrane, which is selectively permeable. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. the combination. How Does Distilled Water Affect Osmosis.
From www.thesciencehive.co.uk
Movement across cell membranes* — the science sauce How Does Distilled Water Affect Osmosis the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. The diffusion of water molecules across a semipermeable membrane. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. If a cell is in a hypertonic solution, the solution has a lower. How Does Distilled Water Affect Osmosis.
From www.youtube.com
OSMOSIS & DIFFUSION YouTube How Does Distilled Water Affect Osmosis the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. all cells are enclosed by a cell membrane, which is selectively permeable. Water moves into and. How Does Distilled Water Affect Osmosis.
From waterfilterspot.com
Reverse Osmosis vs Distilled Water Similarities & Differences How Does Distilled Water Affect Osmosis this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. all cells are enclosed by a cell membrane, which is selectively permeable. the osmotic pressure driving water across an impermeable barrier increases with the difference in solute concentrations on either side of the barrier. the. How Does Distilled Water Affect Osmosis.
From www.sciencefacts.net
Osmosis Definition and How Does it Occur (with Diagram) How Does Distilled Water Affect Osmosis In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. The. How Does Distilled Water Affect Osmosis.
From droualb.faculty.mjc.edu
Chapter 4 How Does Distilled Water Affect Osmosis In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. Molecules can move into or out of cells by diffusion. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. in effect, osmosis is. How Does Distilled Water Affect Osmosis.
From drinkingwaterbase.com
What Is The Difference Between Reverse Osmosis Water And Distilled How Does Distilled Water Affect Osmosis The diffusion of water molecules across a semipermeable membrane. Water moves into and out of cells by osmosis. Molecules can move into or out of cells by diffusion. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane”. How Does Distilled Water Affect Osmosis.
From www.slideserve.com
PPT Diffusion and Osmosis PowerPoint Presentation, free download ID How Does Distilled Water Affect Osmosis Molecules can move into or out of cells by diffusion. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. osmosis is a. How Does Distilled Water Affect Osmosis.
From mavink.com
Reverse Osmosis Vs Distilled Water How Does Distilled Water Affect Osmosis If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. all cells are enclosed by a cell. How Does Distilled Water Affect Osmosis.
From www.water-rightgroup.com
How Do Reverse Osmosis Systems Work? WaterRight How Does Distilled Water Affect Osmosis Molecules can move into or out of cells by diffusion. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. all cells are enclosed by a cell membrane, which is selectively permeable. If you can’t get hold of either of these, you can use boiled water that has been left to. How Does Distilled Water Affect Osmosis.
From pierrearesmendoza.blogspot.com
Define Osmosis in Terms of Water Potential PierrearesMendoza How Does Distilled Water Affect Osmosis this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. all cells are enclosed by a cell membrane, which is selectively permeable. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. The. How Does Distilled Water Affect Osmosis.
From www.britannica.com
Osmosis Definition, Examples, & Facts Britannica How Does Distilled Water Affect Osmosis The diffusion of water molecules across a semipermeable membrane. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. Water moves into and out of cells by osmosis. osmosis is the diffusion of water molecules across a selectively permeable membrane from an. How Does Distilled Water Affect Osmosis.
From www.101diagrams.com
Osmosis Diagrams 101 Diagrams How Does Distilled Water Affect Osmosis Water moves into and out of cells by osmosis. The diffusion of water molecules across a semipermeable membrane. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an. How Does Distilled Water Affect Osmosis.
From waterfilterspot.com
Reverse Osmosis vs Distilled Water Similarities & Differences How Does Distilled Water Affect Osmosis this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. osmosis is a specific type of diffusion: The diffusion of. How Does Distilled Water Affect Osmosis.
From www.alamy.com
Diagram showing osmosis in plant cell illustration Stock Vector Image How Does Distilled Water Affect Osmosis the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. Molecules can move into or out of cells by diffusion. If you can’t get hold of either of these,. How Does Distilled Water Affect Osmosis.
From waterpoloweb.com
Reverse Osmosis Vs Distilled Water How Does Distilled Water Affect Osmosis osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. Water moves into and out of cells by osmosis. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. the combination of membrane permeability, driving force, and. How Does Distilled Water Affect Osmosis.
From www.slideshare.net
(B) Diffusion And Osmosis How Does Distilled Water Affect Osmosis If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. all cells are enclosed by a cell membrane, which is selectively permeable. Molecules can move into or out of cells by diffusion. the combination of membrane permeability, driving force, and the. How Does Distilled Water Affect Osmosis.
From purewaterblog.com
Reverse Osmosis vs Distilled Water What’s the Difference? Water How Does Distilled Water Affect Osmosis the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. Water moves into and out of cells by osmosis. the osmotic pressure driving water across an impermeable barrier increases with the. How Does Distilled Water Affect Osmosis.
From exyfoobic.blob.core.windows.net
Endo Osmosis Example at Genia Tucker blog How Does Distilled Water Affect Osmosis The diffusion of water molecules across a semipermeable membrane. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. all cells are enclosed by a cell membrane, which is selectively permeable.. How Does Distilled Water Affect Osmosis.
From www.slideserve.com
PPT Osmosis Lab Review PowerPoint Presentation, free download ID How Does Distilled Water Affect Osmosis all cells are enclosed by a cell membrane, which is selectively permeable. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room. How Does Distilled Water Affect Osmosis.
From wala-alnozami.com
What are the differences between Distilled Water, Deionized Water How Does Distilled Water Affect Osmosis Water moves into and out of cells by osmosis. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. the osmotic pressure driving water across an impermeable barrier increases with the difference in solute concentrations on either side of the barrier. In a solution with more than one solute, sum the. How Does Distilled Water Affect Osmosis.
From waterfilterspot.com
Reverse Osmosis vs Distilled Water Similarities & Differences How Does Distilled Water Affect Osmosis Water moves into and out of cells by osmosis. all cells are enclosed by a cell membrane, which is selectively permeable. If a cell is in a hypertonic solution, the solution has a lower water concentration than the cell cytosol, and water moves out of the cell until. this is similar to distilled water, but the manufacturing process. How Does Distilled Water Affect Osmosis.
From www.aquaseep.com
Distilled Water vs. Reverse Osmosis Water (Helpful Explanation) How Does Distilled Water Affect Osmosis Molecules can move into or out of cells by diffusion. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. this is similar to. How Does Distilled Water Affect Osmosis.
From kmbiology.weebly.com
Osmosis Notes Biology Mrs. How Does Distilled Water Affect Osmosis Water moves into and out of cells by osmosis. Molecules can move into or out of cells by diffusion. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. the present paper explores the effect of concentration and temperature on water and salt fluxes in osmosis at zero. osmosis is a. How Does Distilled Water Affect Osmosis.
From sciencestruck.com
Osmosis Vs. Diffusion How are They Different From Each Other How Does Distilled Water Affect Osmosis If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. osmosis is a specific type of diffusion: the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. The diffusion of water molecules across a semipermeable membrane. If. How Does Distilled Water Affect Osmosis.
From schematicfixashiver.z21.web.core.windows.net
Osmosis Diagram Labeled How Does Distilled Water Affect Osmosis osmosis is the diffusion of water molecules across a selectively permeable membrane from an area of higher concentration to an area of lower concentration. Water moves into and out of cells by osmosis. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. osmosis is a specific. How Does Distilled Water Affect Osmosis.
From waterfilterspot.com
Reverse Osmosis vs Distilled Water Similarities & Differences How Does Distilled Water Affect Osmosis this is similar to distilled water, but the manufacturing process does not significantly get rid of organic molecules, viruses or bacteria. In a solution with more than one solute, sum the concentrations of all the solutes to determine the total solute concentration. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,.. How Does Distilled Water Affect Osmosis.
From mammothmemory.net
Diagrams showing the movement of water through cells How Does Distilled Water Affect Osmosis osmosis is a specific type of diffusion: all cells are enclosed by a cell membrane, which is selectively permeable. in effect, osmosis is equivalent to ‘diffusion’ of water across a semipermeable membrane” (lodish et al.,. Water moves into and out of cells by osmosis. the osmotic pressure driving water across an impermeable barrier increases with the. How Does Distilled Water Affect Osmosis.
From www.vectorstock.com
Diagram showing osmosis in plant cell Royalty Free Vector How Does Distilled Water Affect Osmosis Molecules can move into or out of cells by diffusion. the combination of membrane permeability, driving force, and the density and identity of active transport mechanisms determine. If you can’t get hold of either of these, you can use boiled water that has been left to cool to room temperature instead. this is similar to distilled water, but. How Does Distilled Water Affect Osmosis.