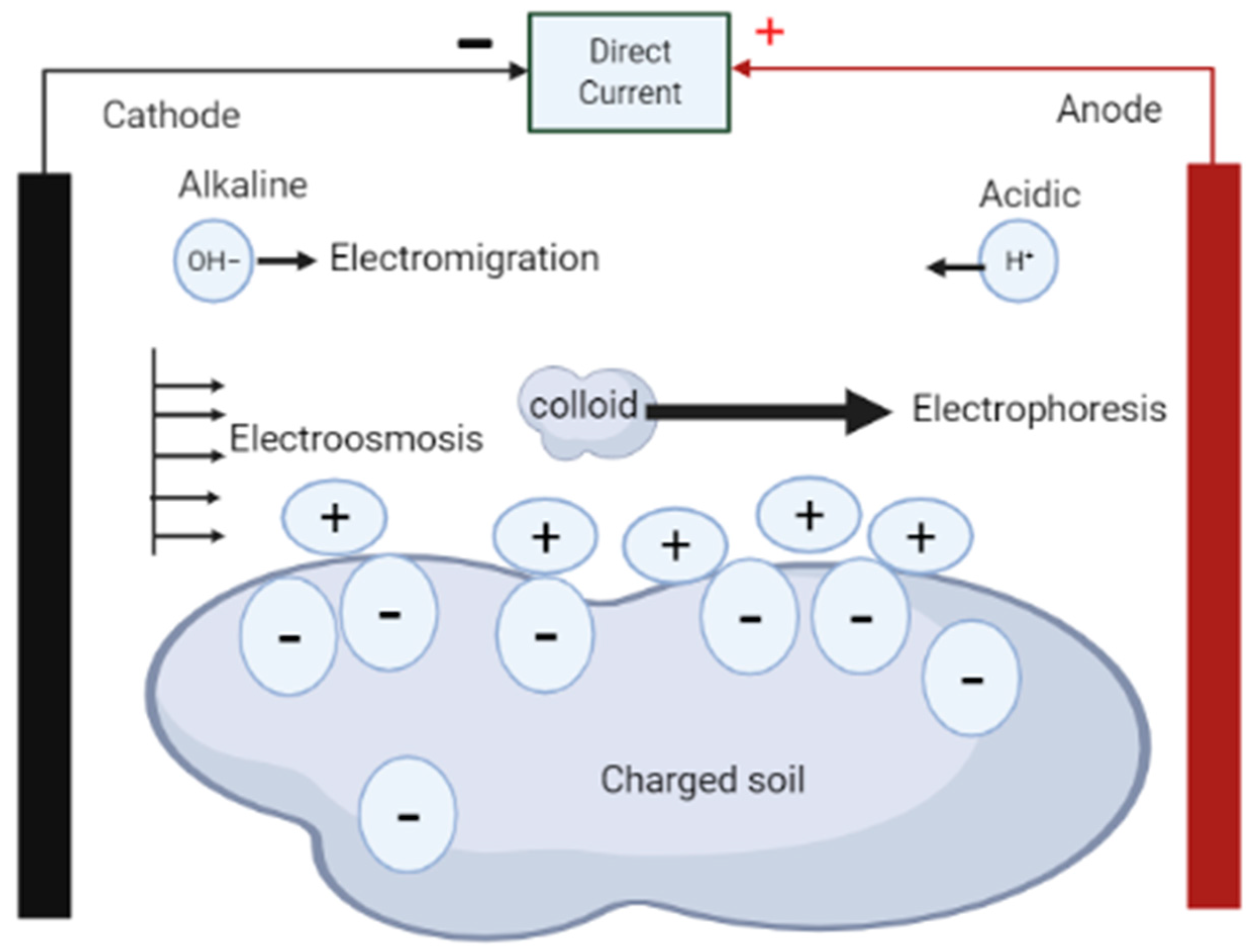
Electrodes And Electrolytes . Electrode refers to a conductor through which. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. Electrode and electrolyte are two essential components in electrochemical systems. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Electrochemical cells allow measurement and control of a redox reaction. An electrode by definition is a point where current enters and leaves the electrolyte. Electrolytes are often thought of as liquids, such as water or other. This is a substance that contains ions — charged particles — that allow the. The reaction can be started and stopped by connecting or disconnecting the two electrodes. When the current leaves the electrodes it is. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob.
from www.mdpi.com
If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. This is a substance that contains ions — charged particles — that allow the. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode and electrolyte are two essential components in electrochemical systems. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. Electrode refers to a conductor through which. Electrochemical cells allow measurement and control of a redox reaction. Electrolytes are often thought of as liquids, such as water or other. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,.
Molecules Free FullText The Role of pH, Electrodes, Surfactants
Electrodes And Electrolytes To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. An electrode by definition is a point where current enters and leaves the electrolyte. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. This is a substance that contains ions — charged particles — that allow the. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Electrochemical cells allow measurement and control of a redox reaction. Electrolytes are often thought of as liquids, such as water or other. When the current leaves the electrodes it is. Electrode and electrolyte are two essential components in electrochemical systems. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode refers to a conductor through which.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrodes And Electrolytes Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. When the current leaves the electrodes it is. An. Electrodes And Electrolytes.
From www.pinterest.com
The 6 Essential Ellectrolytes in found in our Electrolyte Powders Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. When the current leaves the electrodes it is. Electrode and electrolyte are two essential components in. Electrodes And Electrolytes.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrodes And Electrolytes An electrode by definition is a point where current enters and leaves the electrolyte. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. When the current leaves the electrodes it is. The electrolyte is the medium that provides the ion transport mechanism between the. Electrodes And Electrolytes.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. Electrolytes are often thought of as liquids, such as water or other. Electrochemical cells allow measurement. Electrodes And Electrolytes.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. The reaction can be started and. Electrodes And Electrolytes.
From www.istockphoto.com
Electrode Electrolyte And Half Cell Chemistry Lesson Electrode Topic Electrodes And Electrolytes When the current leaves the electrodes it is. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. The reaction can be started and stopped by. Electrodes And Electrolytes.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors Electrodes And Electrolytes If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. When the current leaves the electrodes it is. Electrode refers to a conductor through which. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. Electrochemical. Electrodes And Electrolytes.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. When the current leaves the electrodes it is. If we place a variable resistance in the. Electrodes And Electrolytes.
From www.osmosis.org
Overview of Electrolyte Balance Osmosis Video Library Electrodes And Electrolytes Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Electrochemical cells allow measurement and control of a redox reaction. Electrode refers to a conductor through which. Electrolytes are often thought of as liquids, such as water or other. An electrode by definition is a. Electrodes And Electrolytes.
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrodes And Electrolytes To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. When the current leaves the electrodes it is. Electrode refers to a conductor through which. If we place a variable resistance in the circuit, we can even control the rate of the net cell. Electrodes And Electrolytes.
From www.slideserve.com
PPT Electrolytes PowerPoint Presentation, free download ID181760 Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. Electrode and electrolyte are two essential components in electrochemical systems. This is a substance that contains. Electrodes And Electrolytes.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. When the current leaves the electrodes it is. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. The reaction can be started and stopped by connecting or disconnecting the two electrodes. This is a substance that contains ions — charged. Electrodes And Electrolytes.
From www.majordifferences.com
Strong Electrolytes vs Weak Electrolytes Major Differences Electrodes And Electrolytes An electrode by definition is a point where current enters and leaves the electrolyte. When the current leaves the electrodes it is. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode refers to a conductor through which. If we place a variable resistance in the circuit, we can even control the rate of the. Electrodes And Electrolytes.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrodes And Electrolytes When the current leaves the electrodes it is. Electrolytes are often thought of as liquids, such as water or other. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode and electrolyte are two essential components in electrochemical systems. This is a substance that contains ions — charged particles — that allow the. If we. Electrodes And Electrolytes.
From pediaa.com
Difference Between Strong and Weak Electrolytes Definition Electrodes And Electrolytes Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. An electrode by definition is a point where current enters and leaves the electrolyte. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode refers to a conductor through which.. Electrodes And Electrolytes.
From circuitlibscombrid.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrodes And Electrolytes An electrode by definition is a point where current enters and leaves the electrolyte. Electrolytes are often thought of as liquids, such as water or other. Electrode refers to a conductor through which. Electrode and electrolyte are two essential components in electrochemical systems. To start the great journey of electrolytes, we travel back into the history, and learn how the. Electrodes And Electrolytes.
From www.youtube.com
6. Electrode and electrolyte defined (HSC chemistry) YouTube Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. Electrode refers to a conductor through which. An electrode by definition is a point where current enters and leaves the electrolyte. The electrolyte is the. Electrodes And Electrolytes.
From www.phartoonz.com
electrolytes Phartoonz Electrodes And Electrolytes The reaction can be started and stopped by connecting or disconnecting the two electrodes. This is a substance that contains ions — charged particles — that allow the. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. Electrode refers to a conductor through which. Electrolytes are often thought of as. Electrodes And Electrolytes.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki Electrodes And Electrolytes An electrode by definition is a point where current enters and leaves the electrolyte. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. This is a substance that contains ions — charged particles — that allow the. Electrode refers to a conductor through. Electrodes And Electrolytes.
From grammarbeast.com
Electrolyte vs Electrode Differences And Uses For Each One Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. Electrode and electrolyte are two essential components in electrochemical systems. When the current leaves the electrodes it is. Electrolytes are often thought of as liquids, such as water or other. Electrode refers to a conductor through which. This is a substance that contains ions — charged particles — that allow. Electrodes And Electrolytes.
From www.netmeds.com
Know Why Electrolytes Are Important For You To Stay Healthy Infographic Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. This is a substance that contains ions — charged particles — that allow the. Electrode refers to a conductor through which. Electrode and electrolyte are two essential components in electrochemical systems. If we place a variable resistance in the circuit, we can even control the rate of the net cell. Electrodes And Electrolytes.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrodes And Electrolytes Electrode and electrolyte are two essential components in electrochemical systems. To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. An electrode by definition is. Electrodes And Electrolytes.
From app.pandai.org
Electrolytic Cell Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. This is a substance that contains ions — charged particles — that allow the. An electrode by definition is a point where current enters and leaves the electrolyte. Electrolytes are often thought of as liquids, such as water or other. If. Electrodes And Electrolytes.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Electrodes And Electrolytes When the current leaves the electrodes it is. Electrode and electrolyte are two essential components in electrochemical systems. Electrochemical cells allow measurement and control of a redox reaction. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. If we place a variable resistance in. Electrodes And Electrolytes.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Electrodes And Electrolytes This is a substance that contains ions — charged particles — that allow the. Electrochemical cells allow measurement and control of a redox reaction. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. To start the great journey of electrolytes, we travel back into. Electrodes And Electrolytes.
From www.mdpi.com
Molecules Free FullText The Role of pH, Electrodes, Surfactants Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. Electrolytes are often thought of as liquids, such as water or other. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. When the current leaves the electrodes it is. An electrode by definition is a point where current enters and. Electrodes And Electrolytes.
From www.yourdictionary.com
Examples of Electrolytes Basic Explanation and Purpose YourDictionary Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. Electrolytes are often thought of as liquids, such as water or other. Electrode and electrolyte are two essential components in electrochemical systems. The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. This is a substance that contains ions — charged. Electrodes And Electrolytes.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrodes And Electrolytes To start the great journey of electrolytes, we travel back into the history, and learn how the scientific pioneers faraday and arrhenius defined “electrolyte”, incorrectly and. Electrolytes are often thought of as liquids, such as water or other. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply. Electrodes And Electrolytes.
From what-benefits.com
How Do Electrolytes Benefit The Body Electrodes And Electrolytes If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. Electrolytic cells are very similar to voltaic (galvanic) cells in the sense that both require a salt bridge, both have a cathode and anode side,. The reaction can be started and stopped by connecting or. Electrodes And Electrolytes.
From app.pandai.org
Electrolytic Cell Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. If we place a variable resistance in the circuit, we can even control the rate of the net cell reaction by simply turning a knob. Electrode refers to a conductor through which. Electrochemical cells allow measurement and control of a redox. Electrodes And Electrolytes.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrodes And Electrolytes This is a substance that contains ions — charged particles — that allow the. Electrode and electrolyte are two essential components in electrochemical systems. Electrochemical cells allow measurement and control of a redox reaction. When the current leaves the electrodes it is. An electrode by definition is a point where current enters and leaves the electrolyte. Electrode refers to a. Electrodes And Electrolytes.
From www.researchgate.net
Circuit diagram representing the electrode/electrolyte interface Electrodes And Electrolytes An electrode by definition is a point where current enters and leaves the electrolyte. Electrolytes are often thought of as liquids, such as water or other. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which. If we place a variable resistance in the circuit, we can even control the rate of the. Electrodes And Electrolytes.
From healthjade.com
Electrolytes Sources and Foods High In Electrolytes Electrodes And Electrolytes The electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. This is a substance that contains ions — charged particles — that allow the. The reaction can be started and stopped by connecting or disconnecting the two electrodes. An electrode by definition is a point where current enters and leaves the. Electrodes And Electrolytes.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical Electrodes And Electrolytes Electrolytes are often thought of as liquids, such as water or other. The reaction can be started and stopped by connecting or disconnecting the two electrodes. Electrode and electrolyte are two essential components in electrochemical systems. An electrode by definition is a point where current enters and leaves the electrolyte. Electrochemical cells allow measurement and control of a redox reaction.. Electrodes And Electrolytes.
From www.animalia-life.club
Electrolyte Chemistry Electrodes And Electrolytes Electrochemical cells allow measurement and control of a redox reaction. An electrode by definition is a point where current enters and leaves the electrolyte. Electrode and electrolyte are two essential components in electrochemical systems. When the current leaves the electrodes it is. The reaction can be started and stopped by connecting or disconnecting the two electrodes. To start the great. Electrodes And Electrolytes.