What Is A Buffer Composed Of . A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes: A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Ions are atoms or molecules that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that maintains the stability of a system’s ph level when adding. What is a buffer composed of? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt.
from slidetodoc.com
A buffer solution resists a change in ph from the addition of a small amount of acid or base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. What is a buffer composed of? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers do so by being composed of certain pairs of solutes: Ions are atoms or molecules that. Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. A buffer is a solution that maintains the stability of a system’s ph level when adding.
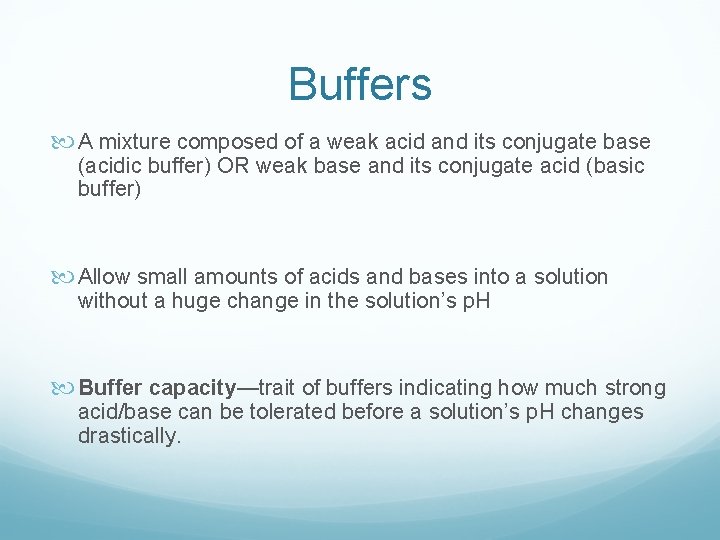
AcidBase Titration Buffers Buffers A mixture composed of
What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Ions are atoms or molecules that. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes:
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution resists. What Is A Buffer Composed Of.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers do. What Is A Buffer Composed Of.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Composed Of A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffers do so by being composed of certain pairs of solutes: Ions are atoms or molecules that. What is. What Is A Buffer Composed Of.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Ions are atoms or molecules that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base.. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: Ions are atoms or molecules that. What is a buffer composed of? A buffer is a solution that maintains the stability of a system’s ph level when adding. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either a. What Is A Buffer Composed Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Composed Of A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the stability of a system’s ph level when adding. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that weak acid. What Is A Buffer Composed Of.
From chem.libretexts.org
10.6 Buffers Chemistry LibreTexts What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: A buffer solution resists a change in ph from the addition of a small amount of acid or base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: A buffer is a solution that. What Is A Buffer Composed Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Composed Of A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Composed Of Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Buffers do so by being composed of certain pairs of solutes: A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes:. What Is A Buffer Composed Of.
From www.slideshare.net
Buffer system What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. The mechanism involves a buffer, a solution that resists. What Is A Buffer Composed Of.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer Composed Of Ions are atoms or molecules that. Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers do so by being. What Is A Buffer Composed Of.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer Composed Of Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. What is a buffer composed of? Buffer, in chemistry, solution usually containing an acid and a. What Is A Buffer Composed Of.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Composed Of Ions are atoms or molecules that. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends. What Is A Buffer Composed Of.
From klafzqach.blob.core.windows.net
What Does A Buffer Do In Chemistry at Lura Laughlin blog What Is A Buffer Composed Of Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution resists a change in. What Is A Buffer Composed Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. What is a buffer composed of? Ions are atoms or molecules that. A buffer solution is one in which the ph of the solution is resistant. What Is A Buffer Composed Of.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is A Buffer Composed Of Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers do so by being composed of certain pairs of solutes: A. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. The mechanism involves a buffer, a solution that resists dramatic changes in ph. What is a buffer composed of? Buffers do. What Is A Buffer Composed Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Composed Of A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Ions are atoms or molecules that. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffer, in chemistry, solution usually containing an acid and a. What Is A Buffer Composed Of.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer Composed Of A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Ions are atoms or molecules that. Buffers do so by being composed of certain pairs of solutes: Buffers do so by being composed of certain pairs of solutes: A buffer solution resists a change in. What Is A Buffer Composed Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Composed Of The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Ions are atoms or molecules that. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. What is a buffer composed of? Ions are atoms or molecules that. Buffers do so by being composed of certain pairs of solutes: A buffer. What Is A Buffer Composed Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Composed Of Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding. What is a buffer composed of? Ions are atoms or molecules that. A buffer solution is one in which the ph. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation, free download What Is A Buffer Composed Of Ions are atoms or molecules that. Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? A buffer is a solution that maintains the stability of a system’s ph level when adding. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution resists a change in ph. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Ions are atoms or molecules that. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution that. What Is A Buffer Composed Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Composed Of What is a buffer composed of? The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains. What Is A Buffer Composed Of.
From cedggslh.blob.core.windows.net
What Is A Buffer How Does It Form at Lloyd Limon blog What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Buffers do so by being composed of certain pairs of solutes: The mechanism involves a buffer, a solution. What Is A Buffer Composed Of.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Composed Of The mechanism involves a buffer, a solution that resists dramatic changes in ph. Ions are atoms or molecules that. A buffer is a solution that maintains the stability of a system’s ph level when adding. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a. What Is A Buffer Composed Of.
From 2012books.lardbucket.org
Buffers What Is A Buffer Composed Of Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers do so by being composed of certain pairs of solutes: Buffers do so by being composed of certain pairs of solutes: What is a buffer composed of? The mechanism involves a buffer, a solution that resists. What Is A Buffer Composed Of.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Composed Of A buffer is a solution that maintains the stability of a system’s ph level when adding. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base.. What Is A Buffer Composed Of.
From exyeghjzx.blob.core.windows.net
What Are The Types Of Buffers at Virginia Saunders blog What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. A buffer is a solution that maintains the stability of a system’s ph level when adding. What is a buffer composed of? A buffer solution resists. What Is A Buffer Composed Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. What is a buffer composed of? Buffers do so by being composed of certain pairs of solutes: A buffer solution is one in which the ph. What Is A Buffer Composed Of.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Composed Of Ions are atoms or molecules that. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. What is a buffer composed of? A buffer solution resists a change. What Is A Buffer Composed Of.
From slidetodoc.com
AcidBase Titration Buffers Buffers A mixture composed of What Is A Buffer Composed Of The mechanism involves a buffer, a solution that resists dramatic changes in ph. Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. What is a buffer composed. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Is A Buffer Composed Of The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak base plus a salt. Ions are atoms or molecules that. A buffer solution resists a change in ph from the addition. What Is A Buffer Composed Of.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is A Buffer Composed Of Buffers do so by being composed of certain pairs of solutes: Buffer, in chemistry, solution usually containing an acid and a base, or a salt, that tends to maintain a constant hydrogen ion concentration. Buffers do so by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid or a weak. What Is A Buffer Composed Of.