What Was The Purpose Of Calculating The Calorimeter Constant . It's the amount of heat. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. We need to find the difference between the heat lost by the hot water when it droped. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. what is the calorimeter constant? the calibration gives you a number called the calorimeter constant.
from studyadvertiser.z21.web.core.windows.net
to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. We need to find the difference between the heat lost by the hot water when it droped. what is the calorimeter constant? calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. the calibration gives you a number called the calorimeter constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. It's the amount of heat.
How To Use A Calorimeter Stepbystep
What Was The Purpose Of Calculating The Calorimeter Constant to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. We need to find the difference between the heat lost by the hot water when it droped. the calibration gives you a number called the calorimeter constant. It's the amount of heat. what is the calorimeter constant? calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure What Was The Purpose Of Calculating The Calorimeter Constant calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. what is the calorimeter constant? the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry, is used to. What Was The Purpose Of Calculating The Calorimeter Constant.
From dxohkumho.blob.core.windows.net
Calorimeter Constant Example Problems at Geraldine Agnew blog What Was The Purpose Of Calculating The Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. the calibration gives you a number called the calorimeter constant. It's the amount of heat. what is the calorimeter constant? to. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry What Was The Purpose Of Calculating The Calorimeter Constant the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. what is the calorimeter constant? calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed. What Was The Purpose Of Calculating The Calorimeter Constant.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog What Was The Purpose Of Calculating The Calorimeter Constant constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. It's the amount of heat. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained. What Was The Purpose Of Calculating The Calorimeter Constant.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses What Was The Purpose Of Calculating The Calorimeter Constant to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. It's the amount of heat. the calibration gives you a number called the calorimeter constant. what is the calorimeter constant? the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket,. What Was The Purpose Of Calculating The Calorimeter Constant.
From studymarxianism.z21.web.core.windows.net
How To Calculate Calorimeter What Was The Purpose Of Calculating The Calorimeter Constant what is the calorimeter constant? constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. to make sure you get accurate results you need to calculate the calorimeter constant, which. What Was The Purpose Of Calculating The Calorimeter Constant.
From saylordotorg.github.io
Calorimetry What Was The Purpose Of Calculating The Calorimeter Constant the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. the calibration gives you a number called the calorimeter constant. constant volume calorimetry, also know as. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube What Was The Purpose Of Calculating The Calorimeter Constant It's the amount of heat. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the calibration gives you a number called the calorimeter constant. the. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube What Was The Purpose Of Calculating The Calorimeter Constant the calibration gives you a number called the calorimeter constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. what is the calorimeter constant? It's the amount. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry What Was The Purpose Of Calculating The Calorimeter Constant constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the calibration gives you a number called the calorimeter constant. We need to find the difference between the heat lost by the hot water when it droped. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube What Was The Purpose Of Calculating The Calorimeter Constant constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. It's the amount of heat. the calibration gives you a number called the calorimeter constant. what is the calorimeter constant?. What Was The Purpose Of Calculating The Calorimeter Constant.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog What Was The Purpose Of Calculating The Calorimeter Constant calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. We need to find the difference between the heat lost by the hot water when it droped. . What Was The Purpose Of Calculating The Calorimeter Constant.
From exomnzeqe.blob.core.windows.net
Calorimeter Constant Units at Meghan Faulkner blog What Was The Purpose Of Calculating The Calorimeter Constant calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. what is the calorimeter constant? It's the amount of heat. the calibration gives you a number. What Was The Purpose Of Calculating The Calorimeter Constant.
From exovxxbwq.blob.core.windows.net
Calorimeter In Chemical Engineering at Elizabeth Hodgson blog What Was The Purpose Of Calculating The Calorimeter Constant the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. the calibration gives you a number called the calorimeter constant. It's the amount of heat. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. to make sure you get. What Was The Purpose Of Calculating The Calorimeter Constant.
From 2012books.lardbucket.org
Calorimetry What Was The Purpose Of Calculating The Calorimeter Constant the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. We need to. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizunderfires.z21.web.core.windows.net
Units Of Calorimeter Constant What Was The Purpose Of Calculating The Calorimeter Constant It's the amount of heat. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. We need to find the difference between the heat lost by the hot water when it droped. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry What Was The Purpose Of Calculating The Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. It's the amount of heat. what is the calorimeter constant? the calorimeter constant is the net heat capacity of the bomb, the. What Was The Purpose Of Calculating The Calorimeter Constant.
From slideplayer.com
Chapter 17 Thermochemistry 17.2 Measuring and Expressing ppt download What Was The Purpose Of Calculating The Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calorimeter. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.numerade.com
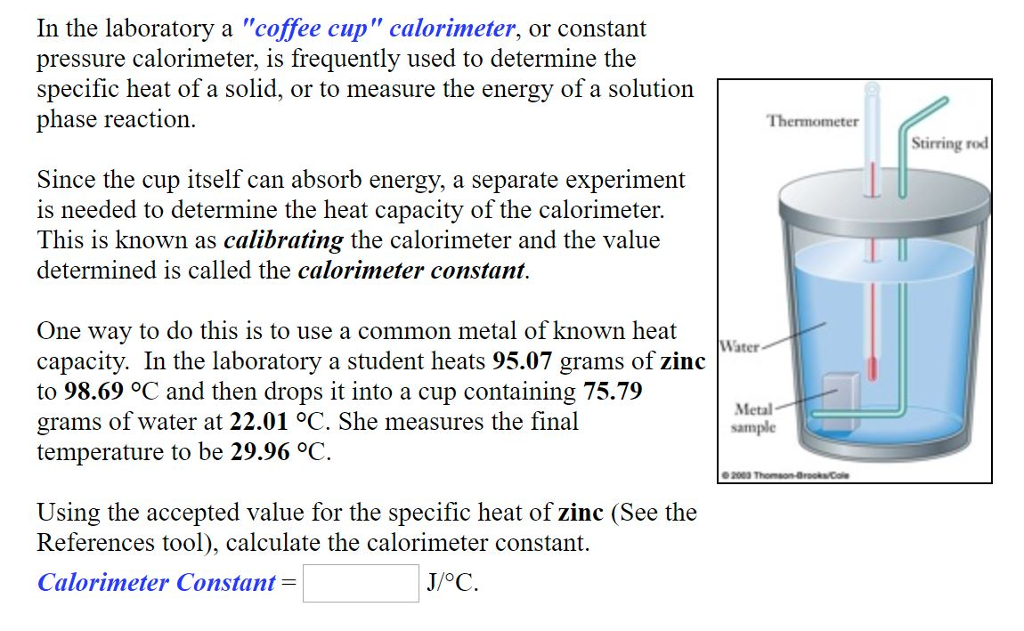
SOLVED In the laboratory a "coffee cup" calorimeter, or constant What Was The Purpose Of Calculating The Calorimeter Constant what is the calorimeter constant? the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. to make sure you get accurate results you need to calculate the calorimeter constant, which. What Was The Purpose Of Calculating The Calorimeter Constant.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter What Was The Purpose Of Calculating The Calorimeter Constant the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. what is the calorimeter constant? calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. We need to find the difference between the heat lost by the. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizdbmonarchist.z21.web.core.windows.net
How To Calculate Calorimeter What Was The Purpose Of Calculating The Calorimeter Constant the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. It's the amount of heat. what is the calorimeter constant? the calibration gives you a number called the. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
Calorimetry calculation YouTube What Was The Purpose Of Calculating The Calorimeter Constant We need to find the difference between the heat lost by the hot water when it droped. It's the amount of heat. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket,. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.numerade.com
SOLVED Text Calculate calorimeter constant CALORIMETRY HEAT CAPACITY What Was The Purpose Of Calculating The Calorimeter Constant the calibration gives you a number called the calorimeter constant. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. We need to find the difference between the heat lost by. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube What Was The Purpose Of Calculating The Calorimeter Constant the calibration gives you a number called the calorimeter constant. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. It's the amount of heat. what is the calorimeter constant? to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Calculator Online What Was The Purpose Of Calculating The Calorimeter Constant constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. . What Was The Purpose Of Calculating The Calorimeter Constant.
From quizunderfires.z21.web.core.windows.net
How To Determine Calorimeter Constant What Was The Purpose Of Calculating The Calorimeter Constant the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the calibration gives you a number called the calorimeter constant. what is the calorimeter constant? the calorimeter constant is. What Was The Purpose Of Calculating The Calorimeter Constant.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep What Was The Purpose Of Calculating The Calorimeter Constant the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Was The Purpose Of Calculating The Calorimeter Constant the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. It's the amount of heat. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizunderfires.z21.web.core.windows.net
Calorimeter Constant Calculator Online What Was The Purpose Of Calculating The Calorimeter Constant calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. We need to find the difference between the heat lost by the hot water when it droped. . What Was The Purpose Of Calculating The Calorimeter Constant.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Was The Purpose Of Calculating The Calorimeter Constant to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calibration gives you a number called the calorimeter constant. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. constant volume calorimetry, also know as bomb calorimetry,. What Was The Purpose Of Calculating The Calorimeter Constant.
From quizunderfires.z21.web.core.windows.net
Units Of Calorimeter Constant What Was The Purpose Of Calculating The Calorimeter Constant what is the calorimeter constant? the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. It's the amount of heat. the formula for calculating the calorimeter. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.chegg.com
Solved Lab Data How to calculate the calorimeter constant What Was The Purpose Of Calculating The Calorimeter Constant constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. the formula for calculating the calorimeter constant (c), also known as calorimeter's heat capacity, is obtained from the. It's the amount of heat. We need to find the difference between the heat lost by the hot water when it droped.. What Was The Purpose Of Calculating The Calorimeter Constant.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog What Was The Purpose Of Calculating The Calorimeter Constant the calibration gives you a number called the calorimeter constant. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at. We need to find the. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.numerade.com
SOLVED In the laboratory, a "coffee cup calorimeter" or constant What Was The Purpose Of Calculating The Calorimeter Constant to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. what is the calorimeter constant? It's the amount of heat. the calibration gives you a number called the. What Was The Purpose Of Calculating The Calorimeter Constant.
From www.thetechedvocate.org
How to calculate calorimeter constant The Tech Edvocate What Was The Purpose Of Calculating The Calorimeter Constant the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. to make sure you get accurate results you need to calculate the calorimeter constant, which is the calorimeter's heat capacitance. We need to find the difference between the heat lost by the hot water when it droped. It's the amount. What Was The Purpose Of Calculating The Calorimeter Constant.