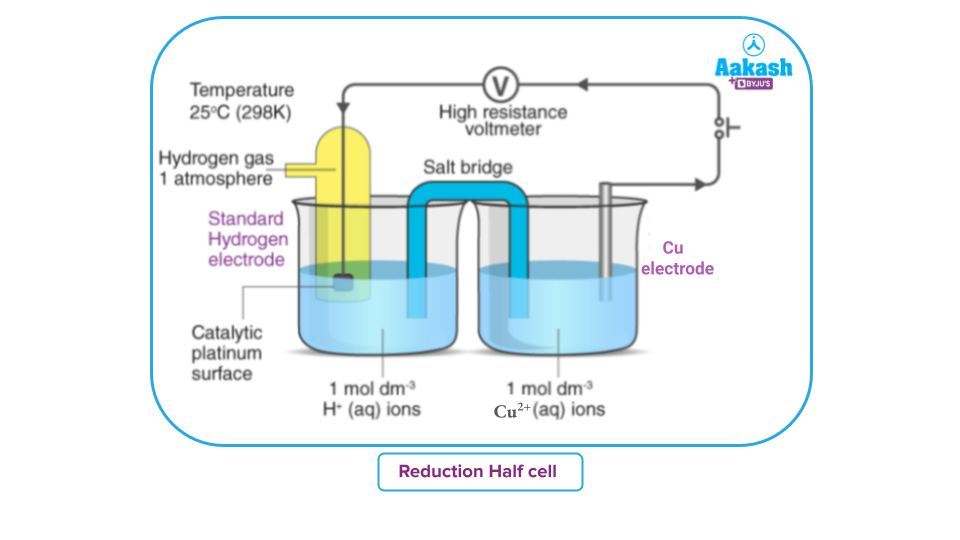
Standard Hydrogen Electrode Practice Problems . Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of:
from www.aakash.ac.in
The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. Calculate the ph needed for a standard hydrogen electrode to have an.
Electrode Potential and Standard Electrode Potential Definition
Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The following practice problems are to assist in your mastery of the topic of electrochemistry. Your answer should include details of:
From www.numerade.com
SOLVED A) Consider the following galvanic cell with a standard Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed. Standard Hydrogen Electrode Practice Problems.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she). Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVED(a) Write the halfreaction that occurs at a hydrogen electrode Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.youtube.com
Calculate the potential of hydrogen electrode in contact with a Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. Your answer should include details of: The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.aakash.ac.in
Electrode Potential and Standard Electrode Potential Definition Standard Hydrogen Electrode Practice Problems Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed. Standard Hydrogen Electrode Practice Problems.
From pubs.acs.org
Absolute Potential of the Standard Hydrogen Electrode and the Problem Standard Hydrogen Electrode Practice Problems Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVED Explain Standard Hydrogen Electrode (SHE) or Normal Hydrogen Standard Hydrogen Electrode Practice Problems Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.youtube.com
PROBLEM ON HYDROGEN ELECTRODE. Problem in electrochemistry YouTube Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist in your mastery of the topic of electrochemistry. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has. Standard Hydrogen Electrode Practice Problems.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: Calculate the ph needed. Standard Hydrogen Electrode Practice Problems.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVED Consider the galvanic cell shown with standard hydrogen Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she). Standard Hydrogen Electrode Practice Problems.
From slidetodoc.com
Standard Reference Electrode Standard Hydrogen Electrode SHE SHE Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under. Standard Hydrogen Electrode Practice Problems.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Standard Hydrogen Electrode Practice Problems Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Practice Problems Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVED In the following cell, A is a standard Sn2tISn electrode Standard Hydrogen Electrode Practice Problems Calculate the ph needed for a standard hydrogen electrode to have an. Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.chegg.com
Solved The standard hydrogen electrode (SHE) consists of a Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: Calculate the ph needed. Standard Hydrogen Electrode Practice Problems.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Standard Hydrogen Electrode Practice Problems Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The following practice problems are to assist in your mastery of the topic. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVEDDraw a diagram of a standard hydrogen electrode and describe how Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Standard Hydrogen Electrode Practice Problems Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Practice Problems Your answer should include details of: The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she). Standard Hydrogen Electrode Practice Problems.
From www.vrogue.co
Hydrogen Standard Hydrogen Electrode vrogue.co Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.chegg.com
Solved to what pH should you adjust a standard hydrogen Standard Hydrogen Electrode Practice Problems Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.studypool.com
SOLUTION Standard hydrogen electrode electrochemistry class 12 Standard Hydrogen Electrode Practice Problems Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.aakash.ac.in
Electrode Potential and Standard Electrode Potential Definition Standard Hydrogen Electrode Practice Problems Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has. Standard Hydrogen Electrode Practice Problems.
From byjus.com
What is standard hydrogen electrode? Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic. Standard Hydrogen Electrode Practice Problems.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Practice Problems Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVED An electrochemical cell consists of a standard hydrogen Standard Hydrogen Electrode Practice Problems The following practice problems are to assist in your mastery of the topic of electrochemistry. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The potential of the standard hydrogen electrode (she). Standard Hydrogen Electrode Practice Problems.
From www.doubtnut.com
When connected to a Standard Hydrogen Electrode (SHE) electrons flow f Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From www.numerade.com
SOLVEDDraw a diagram of a standard hydrogen electrode and describe how Standard Hydrogen Electrode Practice Problems Your answer should include details of: Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic of electrochemistry. The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed. Standard Hydrogen Electrode Practice Problems.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Practice Problems The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. Your answer should include details of: The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The following practice problems are to assist. Standard Hydrogen Electrode Practice Problems.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Practice Problems Your answer should include details of: The following practice problems are to assist in your mastery of the topic of electrochemistry. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The potential of the standard hydrogen electrode (she). Standard Hydrogen Electrode Practice Problems.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Standard Hydrogen Electrode Practice Problems The potential of the standard hydrogen electrode (she) is defined as 0 v under standard conditions. The redox titration of tin and potassium dichromate (vi) is performed in an electrochemical cell that has a platinum. Calculate the ph needed for a standard hydrogen electrode to have an. The following practice problems are to assist in your mastery of the topic. Standard Hydrogen Electrode Practice Problems.