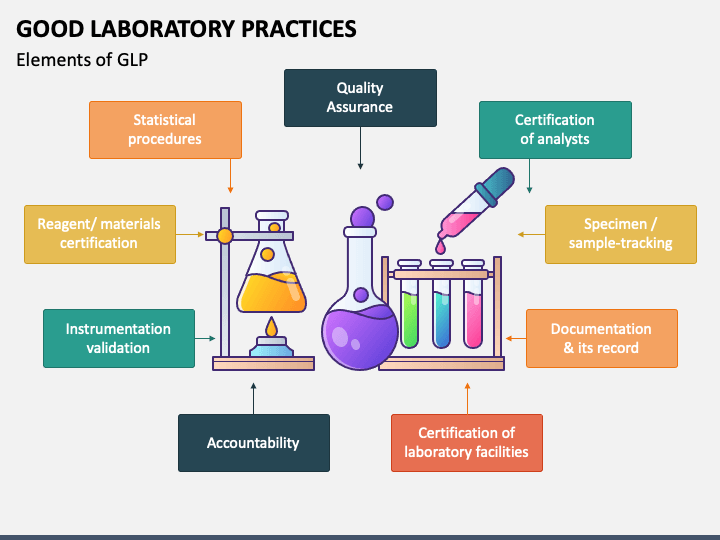
Who Good Clinical Laboratory Practice Guidelines . Ew of recent advances, the icmr has now revised the 2008 document. Compliance with them will allow clinical laboratories to ensure that safety and. The revised guidelines for good clinical laboratory practices 2021 aim to. These gclp guidelines encompass applicable portions of the code of federal regulations. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Clinical laboratory practice (gclp) guidelines. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These gclp guidelines are presented here. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear.
from www.sketchbubble.com
Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Clinical laboratory practice (gclp) guidelines. Ew of recent advances, the icmr has now revised the 2008 document. The revised guidelines for good clinical laboratory practices 2021 aim to. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These gclp guidelines encompass applicable portions of the code of federal regulations. These gclp guidelines are presented here. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Compliance with them will allow clinical laboratories to ensure that safety and.
Good Laboratory Practices PowerPoint and Google Slides Template PPT
Who Good Clinical Laboratory Practice Guidelines The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Compliance with them will allow clinical laboratories to ensure that safety and. These gclp guidelines encompass applicable portions of the code of federal regulations. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. These gclp guidelines are presented here. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Ew of recent advances, the icmr has now revised the 2008 document. The revised guidelines for good clinical laboratory practices 2021 aim to. Clinical laboratory practice (gclp) guidelines. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational.
From www.academia.edu
(PDF) Implementation of Good Clinical Laboratory Practice (GCLP Who Good Clinical Laboratory Practice Guidelines These gclp guidelines are presented here. Compliance with them will allow clinical laboratories to ensure that safety and. The revised guidelines for good clinical laboratory practices 2021 aim to. Ew of recent advances, the icmr has now revised the 2008 document. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned. Who Good Clinical Laboratory Practice Guidelines.
From www.studypool.com
SOLUTION Guidelines for good clinical laboratory practice standards Who Good Clinical Laboratory Practice Guidelines These gclp guidelines encompass applicable portions of the code of federal regulations. Clinical laboratory practice (gclp) guidelines. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples. Who Good Clinical Laboratory Practice Guidelines.
From www.pdffiller.com
Fillable Online Good Clinical Laboratory Practice Standards National Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational.. Who Good Clinical Laboratory Practice Guidelines.
From www.linkedin.com
Understanding the Principles of Good Laboratory Practice (GLP) Who Good Clinical Laboratory Practice Guidelines Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Ew of recent advances, the icmr has now revised the 2008 document. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Compliance with. Who Good Clinical Laboratory Practice Guidelines.
From www.scribd.com
Guidelines for Establishing a Unified Set of Good Clinical Laboratory Who Good Clinical Laboratory Practice Guidelines These gclp guidelines are presented here. Compliance with them will allow clinical laboratories to ensure that safety and. These gclp guidelines encompass applicable portions of the code of federal regulations. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Ew of recent advances,. Who Good Clinical Laboratory Practice Guidelines.
From www.slideserve.com
PPT Good Laboratory Practice PowerPoint Presentation, free download Who Good Clinical Laboratory Practice Guidelines The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These gclp guidelines encompass applicable portions of the code of federal regulations. Clinical laboratory practice (gclp) guidelines. Compliance with them will allow clinical laboratories to ensure that safety and. The revised guidelines for good clinical laboratory practices 2021. Who Good Clinical Laboratory Practice Guidelines.
From www.sketchbubble.com
Good Laboratory Practices PowerPoint and Google Slides Template PPT Who Good Clinical Laboratory Practice Guidelines Clinical laboratory practice (gclp) guidelines. These gclp guidelines encompass applicable portions of the code of federal regulations. Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. These gclp guidelines are presented. Who Good Clinical Laboratory Practice Guidelines.
From www.semanticscholar.org
Figure 3 from Implementation of Good Clinical Laboratory Practice (GCLP Who Good Clinical Laboratory Practice Guidelines Compliance with them will allow clinical laboratories to ensure that safety and. Clinical laboratory practice (gclp) guidelines. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses. Who Good Clinical Laboratory Practice Guidelines.
From jlabphy.org
A Comparative Review of ICMR, WHO, and EMA Guidelines for Good Clinical Who Good Clinical Laboratory Practice Guidelines Compliance with them will allow clinical laboratories to ensure that safety and. Clinical laboratory practice (gclp) guidelines. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. These gclp guidelines are presented here. The principles of good laboratory practice (glp) define a set of. Who Good Clinical Laboratory Practice Guidelines.
From www.researchgate.net
(PDF) A Comparative Review of ICMR, WHO, and EMA Guidelines for Good Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. These gclp guidelines encompass applicable portions of the code of federal regulations. These gclp guidelines are presented here. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Guideline for good clinical practice (gcp) and the implementation. Who Good Clinical Laboratory Practice Guidelines.
From www.academia.edu
(PDF) Implementing a quality management system using good clinical Who Good Clinical Laboratory Practice Guidelines Compliance with them will allow clinical laboratories to ensure that safety and. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice. Who Good Clinical Laboratory Practice Guidelines.
From docplayer.net
DAIDS Guidelines for Good Clinical Laboratory Practice Standards PDF Who Good Clinical Laboratory Practice Guidelines These gclp guidelines encompass applicable portions of the code of federal regulations. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Clinical laboratory practice (gclp) guidelines. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Compliance. Who Good Clinical Laboratory Practice Guidelines.
From www.researchgate.net
(PDF) A Comparative Review of ICMR, WHO, and EMA Guidelines for Good Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Clinical laboratory practice (gclp) guidelines. Compliance with them will allow clinical laboratories to ensure that safety and. Ew of recent advances, the icmr has now revised the 2008 document.. Who Good Clinical Laboratory Practice Guidelines.
From dokumen.tips
(PDF) Icmr Guidelines for Good clinical Laboratory Practices DOKUMEN.TIPS Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. These gclp guidelines are presented here. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of. Who Good Clinical Laboratory Practice Guidelines.
From dokumen.tips
(PDF) Icmr Guidelines for Good clinical Laboratory Practices Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. These gclp guidelines are presented here. Clinical laboratory practice (gclp) guidelines. The revised guidelines for good clinical laboratory practices 2021 aim to. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Compliance with them will allow clinical. Who Good Clinical Laboratory Practice Guidelines.
From www.studypool.com
SOLUTION Guidelines for good clinical laboratory practice standards Who Good Clinical Laboratory Practice Guidelines The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These gclp guidelines are presented here. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Ew of recent advances, the icmr. Who Good Clinical Laboratory Practice Guidelines.
From microbiologyinfo.com
The Ultimate Guide to Good Laboratory Practices (GLP) Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. These gclp guidelines are presented here. These gclp guidelines encompass applicable portions of the code of federal regulations. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Guideline for good clinical. Who Good Clinical Laboratory Practice Guidelines.
From www.slideserve.com
PPT Good Laboratory Practice PowerPoint Presentation, free download Who Good Clinical Laboratory Practice Guidelines The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. The revised guidelines for good clinical laboratory practices 2021 aim to. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Ew of recent advances, the icmr has. Who Good Clinical Laboratory Practice Guidelines.
From www.semanticscholar.org
Figure 1 from Implementation of Good Clinical Laboratory Practice (GCLP Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles. Who Good Clinical Laboratory Practice Guidelines.
From www.slideserve.com
PPT Good Clinical Practice Standards PowerPoint Presentation, free Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Clinical laboratory practice. Who Good Clinical Laboratory Practice Guidelines.
From www.studocu.com
GCLP Guidelines 2020 Final ICMR Guidelines for Good Clinical Who Good Clinical Laboratory Practice Guidelines Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Clinical laboratory practice (gclp) guidelines. Ew of recent advances, the icmr has now revised the 2008 document. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These. Who Good Clinical Laboratory Practice Guidelines.
From www.researchgate.net
(PDF) Implementing a quality management system using good clinical Who Good Clinical Laboratory Practice Guidelines Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Clinical laboratory practice (gclp) guidelines. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. The revised guidelines for good clinical laboratory practices 2021. Who Good Clinical Laboratory Practice Guidelines.
From www.academia.edu
(PDF) Evaluation and on Good Clinical Laboratory Who Good Clinical Laboratory Practice Guidelines Compliance with them will allow clinical laboratories to ensure that safety and. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. These gclp guidelines are presented here. These gclp guidelines encompass applicable portions of the code of federal regulations. Guideline for good clinical. Who Good Clinical Laboratory Practice Guidelines.
From www.academia.edu
(PDF) Guidelines on good clinical laboratory practice Bridging Who Good Clinical Laboratory Practice Guidelines Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Ew of recent advances, the icmr has now revised the 2008 document. These gclp guidelines are presented here. Clinical laboratory practice (gclp) guidelines. Compliance with them will allow clinical laboratories to ensure that safety and. The principles of good laboratory. Who Good Clinical Laboratory Practice Guidelines.
From www.researchgate.net
Principles of good laboratory practice (GLP) Download Scientific Diagram Who Good Clinical Laboratory Practice Guidelines These gclp guidelines encompass applicable portions of the code of federal regulations. Compliance with them will allow clinical laboratories to ensure that safety and. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. The revised guidelines for good clinical laboratory practices 2021 aim to. Clinical laboratory practice (gclp) guidelines.. Who Good Clinical Laboratory Practice Guidelines.
From www.studypool.com
SOLUTION Guidelines for good clinical laboratory practice standards Who Good Clinical Laboratory Practice Guidelines Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. These gclp guidelines encompass applicable portions of the code of federal regulations. Compliance with them will allow clinical laboratories to ensure that safety and. Ew of recent advances, the icmr has now revised the. Who Good Clinical Laboratory Practice Guidelines.
From dokumen.tips
(PDF) GLP (Good Laboratory Practice) Guidelines in Academic · PDF file Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Clinical laboratory practice (gclp) guidelines. These gclp guidelines encompass applicable portions of the code of federal regulations. Guideline for good clinical practice (gcp) and the implementation of. Who Good Clinical Laboratory Practice Guidelines.
From dokumen.tips
(PDF) GUIDELINES FOR GOOD CLINICAL LABORATORY PRACTICE Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. Compliance with them will allow clinical laboratories to ensure that safety and. These gclp guidelines encompass applicable portions of the code of federal regulations. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Clinical laboratory. Who Good Clinical Laboratory Practice Guidelines.
From www.academia.edu
(PDF) Evaluation and on Good Clinical Laboratory Who Good Clinical Laboratory Practice Guidelines These gclp guidelines are presented here. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. Compliance with them will allow clinical laboratories to. Who Good Clinical Laboratory Practice Guidelines.
From www.slideserve.com
PPT Good Laboratory Practice (GLP) PowerPoint Presentation ID6742511 Who Good Clinical Laboratory Practice Guidelines The revised guidelines for good clinical laboratory practices 2021 aim to. Compliance with them will allow clinical laboratories to ensure that safety and. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. These gclp guidelines are presented here. The principles of good laboratory practice (glp) define a set of. Who Good Clinical Laboratory Practice Guidelines.
From andarupm.co.id
GLP Good Laboratory Practice Pengertian, Fungsi dan Prinsipnya Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. The revised guidelines for good clinical laboratory practices 2021 aim to. Compliance with them will allow clinical laboratories to ensure that safety. Who Good Clinical Laboratory Practice Guidelines.
From www.researchgate.net
(PDF) Implementation of Good Clinical Laboratory Practice (GCLP Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. Compliance with them will allow clinical laboratories to ensure that safety and. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice. Who Good Clinical Laboratory Practice Guidelines.
From www.sketchbubble.com
Good Laboratory Practices PowerPoint and Google Slides Template PPT Who Good Clinical Laboratory Practice Guidelines Ew of recent advances, the icmr has now revised the 2008 document. Good clinical laboratory practice (gclp) guidelines describe the application of those good laboratory practice principles that are relevant to the analyses of samples from clinical trials. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational.. Who Good Clinical Laboratory Practice Guidelines.
From www.semanticscholar.org
Figure 2 from Implementation of Good Clinical Laboratory Practice (GCLP Who Good Clinical Laboratory Practice Guidelines Clinical laboratory practice (gclp) guidelines. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. Guideline for good clinical practice (gcp) and the implementation of the european union clinical trials directive (2001/20/ec) are two clear. The revised guidelines for good clinical laboratory practices 2021 aim to. These gclp. Who Good Clinical Laboratory Practice Guidelines.
From pdfslide.net
(PDF) GUIDELINES FOR GOOD CLINICAL LABORATORY PRACTICE Who Good Clinical Laboratory Practice Guidelines These gclp guidelines are presented here. The principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the organisational. These gclp guidelines encompass applicable portions of the code of federal regulations. Ew of recent advances, the icmr has now revised the 2008 document. The revised guidelines for good clinical laboratory practices. Who Good Clinical Laboratory Practice Guidelines.