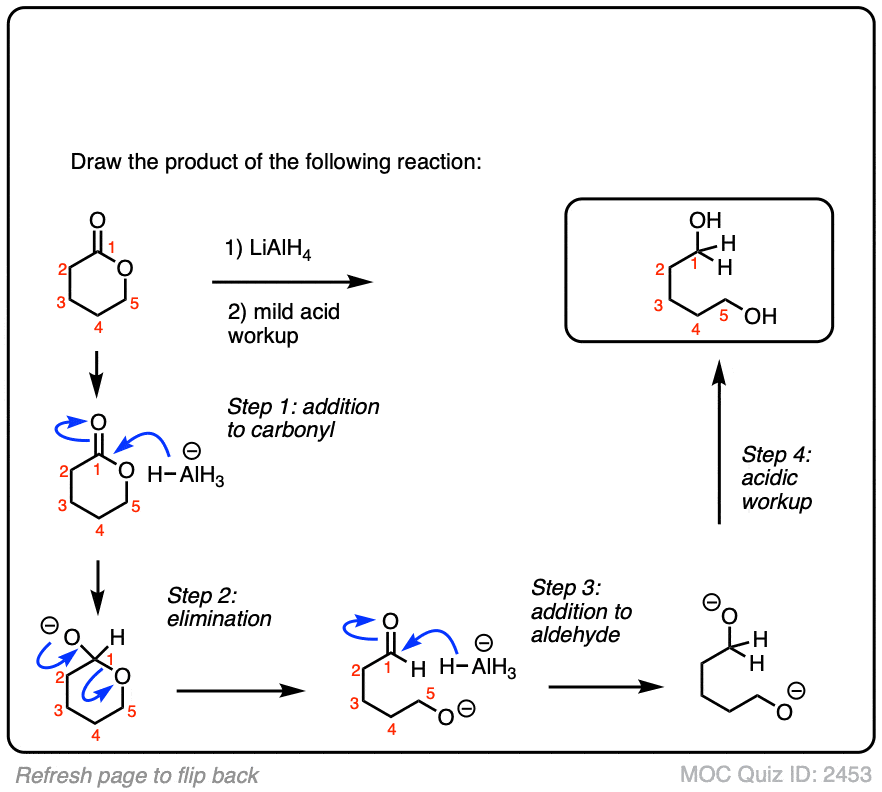
Acetic Acid + Lialh4 . this page looks at the reduction of carboxylic acids to primary alcohols using lithium. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. the main issue is that the al needs to remove its hydride. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol. (a) (b) (c) (d) q2. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4).
from www.masterorganicchemistry.com
As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). With a carboxylic acid and/or an aldehyde, it can. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. (a) (b) (c) (d) q2. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. The correct option is c ethyl alcohol. the main issue is that the al needs to remove its hydride. this page looks at the reduction of carboxylic acids to primary alcohols using lithium.
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives Master Organic
Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). this page looks at the reduction of carboxylic acids to primary alcohols using lithium. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. the main issue is that the al needs to remove its hydride. (a) (b) (c) (d) q2. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol.
From ar.inspiredpencil.com
Lialh4 Mechanism Acetic Acid + Lialh4 this page looks at the reduction of carboxylic acids to primary alcohols using lithium. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). (a) (b) (c) (d) q2. learn about the definition, structure,. Acetic Acid + Lialh4.
From www.doubtnut.com
[Odia] What happens with when acetic acid is heated with LiAlH4 Acetic Acid + Lialh4 The correct option is c ethyl alcohol. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. this page looks at the reduction of carboxylic acids to primary alcohols using lithium. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. (a) (b). Acetic Acid + Lialh4.
From www.masterorganicchemistry.com
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives Master Organic Acetic Acid + Lialh4 carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). the main issue is that the al needs to remove its hydride. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. (a) (b) (c) (d) q2. As refer to image 01 reduction of carboxylic acid or acetic acid by using. Acetic Acid + Lialh4.
From www.numerade.com
SOLVED In each reaction box, place the best reagent and conditions from the list 2) 3) 4) 5 Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. The correct option is c ethyl alcohol. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. With a carboxylic acid and/or an aldehyde, it can. this page looks at the reduction of carboxylic acids to. Acetic Acid + Lialh4.
From en.wikipedia.org
FileAceticacid2Dflat.png Wikipedia Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. (a) (b) (c) (d) q2. The correct option is c ethyl alcohol. As refer to image 01 reduction of carboxylic acid or acetic acid by. Acetic Acid + Lialh4.
From www.numerade.com
SOLVED SOCl2; 2. CH3Br; 3. H3O+ SOCl2; 2. CH3NH2; 3. LiAlH4 Mg; 2. CO2; 3. H3O+ LiAlH4; 2 Acetic Acid + Lialh4 As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. the main issue is that the al needs to remove its hydride. With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol. learn about the definition, structure, properties, preparation, and applications of. Acetic Acid + Lialh4.
From www.vectorstock.com
Formula and model of acetic acid molecule Vector Image Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. With a carboxylic acid and/or an aldehyde, it can. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. (a) (b) (c) (d) q2. As refer to image 01 reduction of carboxylic acid or acetic. Acetic Acid + Lialh4.
From www.alamy.com
Acetic Acid, Structural chemical formula on a white background Stock Photo Alamy Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. With a carboxylic acid and/or an aldehyde, it can. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. The correct option is c ethyl alcohol. the main issue is that the al needs to. Acetic Acid + Lialh4.
From ar.inspiredpencil.com
Liald4 Mechanism Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. The correct option is c ethyl alcohol. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). the main issue is that the al needs to remove its hydride. this page looks at the reduction of carboxylic acids to primary. Acetic Acid + Lialh4.
From www.youtube.com
LiAlH4 converts acetic acid into YouTube Acetic Acid + Lialh4 The correct option is c ethyl alcohol. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). (a) (b) (c) (d) q2. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. With a carboxylic acid and/or an aldehyde, it can. give the aldehyde, ketone, or carboxylic acid (there can be. Acetic Acid + Lialh4.
From byjus.com
Mechanism for reduction of benzoic acid using LiAlH4 Acetic Acid + Lialh4 With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. (a) (b) (c) (d) q2. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. give the aldehyde, ketone,. Acetic Acid + Lialh4.
From www.youtube.com
Reduction of Acid Anhydride using Lithium Aluminium Hydride LiAlH4 for JEE & NEET (JoBLess Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. (a) (b) (c) (d) q2. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. the main issue is. Acetic Acid + Lialh4.
From leah4sci.com
Acetal and Hemiacetal Formation Reaction Mechanism From Aldehydes and Ketones Acetic Acid + Lialh4 carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). With a carboxylic acid and/or an aldehyde, it can. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. The correct option is c ethyl alcohol. As refer to image 01 reduction of carboxylic. Acetic Acid + Lialh4.
From www.pinterest.es
LiAlH4, NaBH4, DIBAL Reduction Summary for Aldehydes, Ketones, Esters and carboxylic Acids Acetic Acid + Lialh4 this page looks at the reduction of carboxylic acids to primary alcohols using lithium. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). With a carboxylic acid and/or an aldehyde, it can. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. The correct option is c ethyl alcohol. . Acetic Acid + Lialh4.
From www.degruyter.com
Synthesis of morphan derivatives with additional substituents in 8position Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. As refer to image 01 reduction of carboxylic acid or acetic. Acetic Acid + Lialh4.
From chemicalforfo.blogspot.com
What Reacts With Acetic Acid Chemical Formula Info Acetic Acid + Lialh4 The correct option is c ethyl alcohol. this page looks at the reduction of carboxylic acids to primary alcohols using lithium. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. With a carboxylic acid and/or an aldehyde, it can. the main issue is that the al needs to remove its hydride. (a) (b). Acetic Acid + Lialh4.
From www.pinterest.cl
The Mechanism of Amide Reduction to Amine by LiAlH4 Organic chemistry mechanisms, Chemistry Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. learn about the definition,. Acetic Acid + Lialh4.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Illustration Illustration of biochemistry, flat Acetic Acid + Lialh4 carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). With a carboxylic acid and/or an aldehyde, it can. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. The correct option is c ethyl alcohol. learn about the definition, structure, properties, preparation, and applications. Acetic Acid + Lialh4.
From www.chemistrysteps.com
LiAlH4 and NaBH4 Carbonyl Reduction Mechanism Chemistry Steps Acetic Acid + Lialh4 The correct option is c ethyl alcohol. With a carboxylic acid and/or an aldehyde, it can. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be. Acetic Acid + Lialh4.
From www.marefa.org
ملفAceticacid2Dskeletal.svg المعرفة Acetic Acid + Lialh4 With a carboxylic acid and/or an aldehyde, it can. the main issue is that the al needs to remove its hydride. The correct option is c ethyl alcohol. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). (a) (b) (c). Acetic Acid + Lialh4.
From www.researchgate.net
Acetic acid conversion to ethanol by LiALh4? ResearchGate Acetic Acid + Lialh4 give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol. carboxylic. Acetic Acid + Lialh4.
From www.pinterest.com
LiAlH4 Ester reduction Mechanism Organic chemistry books, Organic chemistry study, Organic Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. The correct option is c ethyl alcohol. the main issue is that the al needs to remove its hydride. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. As refer to image. Acetic Acid + Lialh4.
From www.youtube.com
Reduction of Carboxylic acid l Acetic acid Ethanol LiAlH4 Organic Chemistry Class12 Acetic Acid + Lialh4 With a carboxylic acid and/or an aldehyde, it can. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. the main issue is that the al needs to remove its hydride. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). (a) (b) (c) (d) q2. The correct option is c. Acetic Acid + Lialh4.
From www.masterorganicchemistry.com
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives Master Organic Acetic Acid + Lialh4 this page looks at the reduction of carboxylic acids to primary alcohols using lithium. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). (a) (b) (c) (d) q2. learn about the. Acetic Acid + Lialh4.
From www.toppr.com
LiAlH4 converts acetic acid into? Acetic Acid + Lialh4 (a) (b) (c) (d) q2. With a carboxylic acid and/or an aldehyde, it can. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). the main issue is that the al needs to remove its hydride. The correct option is c. Acetic Acid + Lialh4.
From www.numerade.com
SOLVED Question 4. Predict the product for each of the following reactions COOH + SOCl2 > ACh Acetic Acid + Lialh4 With a carboxylic acid and/or an aldehyde, it can. (a) (b) (c) (d) q2. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). As refer to image 01 reduction of carboxylic acid or. Acetic Acid + Lialh4.
From www.researchgate.net
Scheme 1 Reagents and conditions a SOCl2, EtOH, reflux, 4 h; b LiAlH4,... Download Scientific Acetic Acid + Lialh4 (a) (b) (c) (d) q2. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. The correct option is c ethyl alcohol. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. With a carboxylic acid and/or an. Acetic Acid + Lialh4.
From www.masterorganicchemistry.com
Lithium Aluminum Hydride (LiAlH4) For Reduction of Carboxylic Acid Derivatives Master Organic Acetic Acid + Lialh4 The correct option is c ethyl alcohol. the main issue is that the al needs to remove its hydride. this page looks at the reduction of carboxylic acids to primary alcohols using lithium. (a) (b) (c) (d) q2. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). With a carboxylic acid and/or an. Acetic Acid + Lialh4.
From www.chegg.com
Solved In each reaction box, place the best reagent and Acetic Acid + Lialh4 carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. With a carboxylic acid and/or an aldehyde, it can. learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. . Acetic Acid + Lialh4.
From www.chemistrysteps.com
Amide Reduction Mechanism by LiAlH4 Chemistry Steps Acetic Acid + Lialh4 With a carboxylic acid and/or an aldehyde, it can. carboxylic acids can be converted to 1o alcohols using lithium aluminum hydride (lialh4). The correct option is c ethyl alcohol. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. As refer to image 01 reduction of carboxylic. Acetic Acid + Lialh4.
From www.doubtnut.com
What is the action of the following reagents on ethanoic acid ? (a) Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. With a carboxylic acid and/or an aldehyde, it can. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. the main issue is that the al needs to remove its hydride. (a) (b). Acetic Acid + Lialh4.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. this page looks at the reduction of carboxylic acids to primary alcohols using lithium. learn about the definition, structure, properties, preparation, and applications of lithium. Acetic Acid + Lialh4.
From brainly.in
LiAlH4 converts acetic acid into(a) Acetaldehyde(b) Ethyl alcohol(c) Ethane(d) Methane Brainly.in Acetic Acid + Lialh4 the main issue is that the al needs to remove its hydride. With a carboxylic acid and/or an aldehyde, it can. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to. Acetic Acid + Lialh4.
From www.toppr.com
LiAlH4 converts acetic acid into? Acetic Acid + Lialh4 give the aldehyde, ketone, or carboxylic acid (there can be multiple answers) that could be reduced to form the following alcohols. With a carboxylic acid and/or an aldehyde, it can. The correct option is c ethyl alcohol. (a) (b) (c) (d) q2. the main issue is that the al needs to remove its hydride. carboxylic acids can. Acetic Acid + Lialh4.
From stock.adobe.com
Molecular structure of acetic acid, 3d rendering Stock Illustration Adobe Stock Acetic Acid + Lialh4 learn about the definition, structure, properties, preparation, and applications of lithium aluminium hydride,. the main issue is that the al needs to remove its hydride. As refer to image 01 reduction of carboxylic acid or acetic acid by using lialh 4 to form 1 alcohol. give the aldehyde, ketone, or carboxylic acid (there can be multiple answers). Acetic Acid + Lialh4.