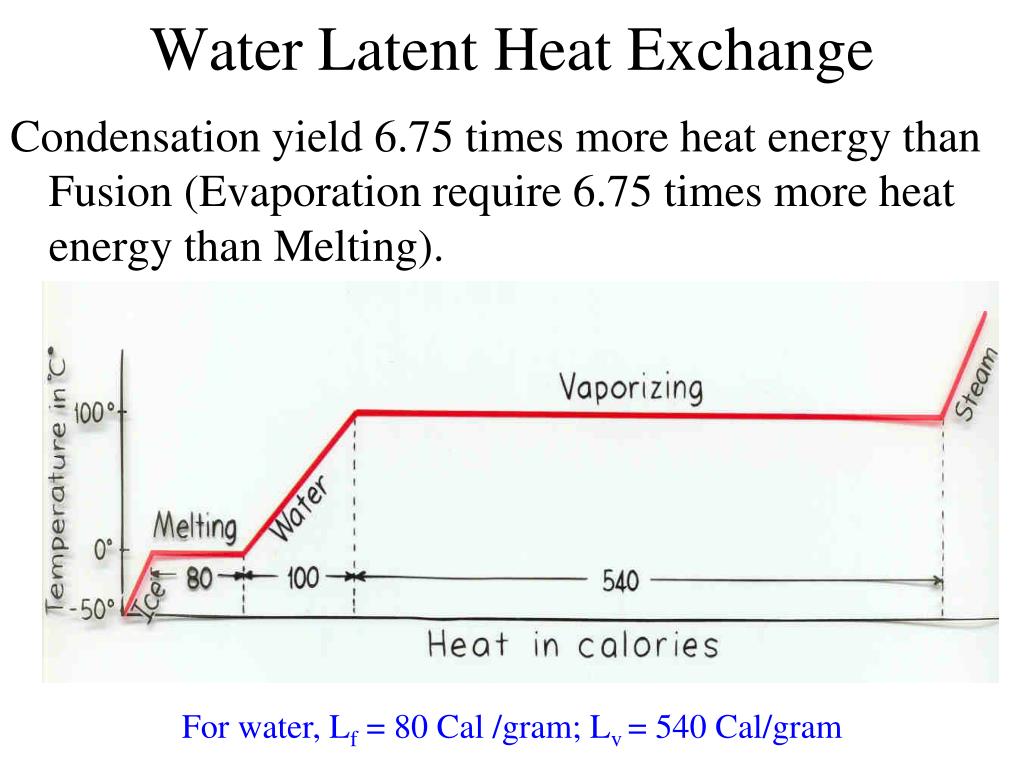
Does Evaporation Require Latent Heat . the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. The heat required to evaporate 10 kg. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\).
from www.slideserve.com
The heat required to evaporate 10 kg. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to.
PPT GEU 0047 Meteorology Lecture 02 Heat Energy PowerPoint Presentation ID4584100
Does Evaporation Require Latent Heat generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. The heat required to evaporate 10 kg. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be.
From www.slideserve.com
PPT AS Geography PowerPoint Presentation, free download ID869666 Does Evaporation Require Latent Heat the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. The heat required to evaporate 10 kg. generally, the latent heat. Does Evaporation Require Latent Heat.
From www.chegg.com
Solved 5. Latent heat of evaporation. You can use the figure Does Evaporation Require Latent Heat generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). but in the gaseous phase the typical distance between molecules are much more larger than the solid and. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Fundamentals of Heat Transfer PowerPoint Presentation, free download ID6182043 Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the latent heat of fusion is the amount of heat needed to cause a phase change between. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT 4.3 SPECIFIC LATENT HEAT PowerPoint Presentation, free download ID6631787 Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. generally, the latent heat of evaporation increases with decreasing temperature, so you need more. Does Evaporation Require Latent Heat.
From www.teachoo.com
How does Evaporation cause cooling? Explain (with Examples) Teachoo Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. The heat required to evaporate 10 kg. the latent heat of fusion is the amount of heat. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation ID3080166 Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. the heat q required to change the phase of a sample of mass m. Does Evaporation Require Latent Heat.
From www.scribd.com
Lecture 5 PDF Latent Heat Evaporation Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. The heat required to evaporate 10 kg. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100. Does Evaporation Require Latent Heat.
From www.nachi.org
Latent Heat of Vaporization Inspection Gallery InterNACHI® Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. The heat required to evaporate 10 kg. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the (latent) heat of vaporization (∆h vap ). Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT What Factors Affect Tropical Cyclogenesis? PowerPoint Presentation ID1586407 Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100. Does Evaporation Require Latent Heat.
From dxovnkvzf.blob.core.windows.net
During Evaporation Latent Heat Is at Shirley Murph blog Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. . Does Evaporation Require Latent Heat.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat Does Evaporation Require Latent Heat the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of. Does Evaporation Require Latent Heat.
From www.coursehero.com
[Solved] Part 1. Latent Heat of Evaporation and Condensation Determine... Course Hero Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is. Does Evaporation Require Latent Heat.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy Does Evaporation Require Latent Heat The heat required to evaporate 10 kg. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. the. Does Evaporation Require Latent Heat.
From dxowsuuoj.blob.core.windows.net
Explain Why The Process Of Evaporation Removes Heat From Your Skin at Grace Hall blog Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. The heat required to evaporate 10 kg. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. but in the gaseous phase the typical distance between molecules are much more larger than the solid. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Chapter 4 Water in the Atmosphere PowerPoint Presentation, free download ID5327476 Does Evaporation Require Latent Heat the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of. Does Evaporation Require Latent Heat.
From www.researchgate.net
Temperature dependence of the latent heat of evaporation of water [43]. Download Scientific Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. The heat required to evaporate 10 kg. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the heat q required to change the phase of a sample. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT METEOROLOGY PowerPoint Presentation, free download ID528399 Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. the latent heat of fusion is the amount. Does Evaporation Require Latent Heat.
From www.tec-science.com
Specific latent heat of condensation tecscience Does Evaporation Require Latent Heat generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. The heat required to evaporate 10 kg. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of mass m is. Does Evaporation Require Latent Heat.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. but in the gaseous phase the typical distance between molecules are much more larger than the solid. Does Evaporation Require Latent Heat.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing Does Evaporation Require Latent Heat The heat required to evaporate 10 kg. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the latent heat of fusion is the amount. Does Evaporation Require Latent Heat.
From dxovnkvzf.blob.core.windows.net
During Evaporation Latent Heat Is at Shirley Murph blog Does Evaporation Require Latent Heat The heat required to evaporate 10 kg. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the heat q required to change the phase of a sample of mass. Does Evaporation Require Latent Heat.
From dxovnkvzf.blob.core.windows.net
During Evaporation Latent Heat Is at Shirley Murph blog Does Evaporation Require Latent Heat generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the. Does Evaporation Require Latent Heat.
From www.youtube.com
Latent heat Latent heat of fusion and vaporization Change of state Formula Chemistry Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. The heat required to evaporate 10 kg. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation,. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT GEU 0047 Meteorology Lecture 02 Heat Energy PowerPoint Presentation ID4584100 Does Evaporation Require Latent Heat the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. The heat required to evaporate 10 kg. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of. Does Evaporation Require Latent Heat.
From www.youtube.com
Latent heat and Evaporation YouTube Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. The heat required to evaporate 10 kg. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT GEU 0047 Meteorology Lecture 02 Heat Energy PowerPoint Presentation ID4584100 Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Chapter 18 Water, Clouds, and Precipitation PowerPoint Presentation ID5622647 Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. The heat required to evaporate 10 kg. the latent heat of fusion is the amount of heat. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Master Streams PowerPoint Presentation, free download ID1597647 Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is. Does Evaporation Require Latent Heat.
From geo.libretexts.org
3.4 Latent Heat and Heat Capacity Geosciences LibreTexts Does Evaporation Require Latent Heat the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy (enthalpy) that must be. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. The heat required to evaporate 10 kg. the heat q required to. Does Evaporation Require Latent Heat.
From www.slideserve.com
PPT Ocean and Atmosphere Coupling Elnino Southern Oscillation PowerPoint Presentation ID Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). The heat required to evaporate 10 kg. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. generally, the latent heat of evaporation increases with decreasing temperature,. Does Evaporation Require Latent Heat.
From www.youtube.com
Lecture 4 Introduction to evaporation and latent heat YouTube Does Evaporation Require Latent Heat the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and. Does Evaporation Require Latent Heat.
From guides.co
States of Matter FD202 Fundamentals of Fire and Combustion on Guides Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. . Does Evaporation Require Latent Heat.
From philschatz.com
Phase Change and Latent Heat · Physics Does Evaporation Require Latent Heat generally, the latent heat of evaporation increases with decreasing temperature, so you need more energy to. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and. Does Evaporation Require Latent Heat.
From www.savemyexams.com
Specific Latent Heat Capacity CIE A Level Physics Revision Notes 2022 Does Evaporation Require Latent Heat the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the latent heat of fusion is the amount of heat needed to cause a phase change between solid and liquid. but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. . Does Evaporation Require Latent Heat.
From www.bpiexamacademy.com
What's the difference between latent and sensible heat on the BPI exam? Take your free BPI Does Evaporation Require Latent Heat but in the gaseous phase the typical distance between molecules are much more larger than the solid and liquid. the heat q required to change the phase of a sample of mass m is given by \(\mathrm{q=ml_f}\) (melting or freezing) and \(\mathrm{q=ml_v}\). the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization. Does Evaporation Require Latent Heat.