Weights Definition Chemistry . In other words, it is the sum of the atomic masses of. Molecular weight is used in chemistry to determine stoichiometry in chemical. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Since 1961 the standard unit. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The molecular weight is the total number of protons and neutrons in a compound. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. The relative masses of the atoms are usually referred to as atomic weights. Their values are in the table of atomic weights,. For example, water (h 2 o) has a formula weight of:.
from collegedunia.com
Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Their values are in the table of atomic weights,. The molecular weight is the total number of protons and neutrons in a compound. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit. The relative masses of the atoms are usually referred to as atomic weights. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Molecular weight is used in chemistry to determine stoichiometry in chemical.
Weight Formula Definition, Mass & Solved Examples
Weights Definition Chemistry The molecular weight is the total number of protons and neutrons in a compound. The molecular weight is the total number of protons and neutrons in a compound. Their values are in the table of atomic weights,. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Molecular weight is used in chemistry to determine stoichiometry in chemical. In other words, it is the sum of the atomic masses of. For example, water (h 2 o) has a formula weight of:. The relative masses of the atoms are usually referred to as atomic weights. Since 1961 the standard unit. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively.
From pediaa.com
Difference Between Molar Mass and Molecular Weight Definition Weights Definition Chemistry In other words, it is the sum of the atomic masses of. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring. Weights Definition Chemistry.
From www.thoughtco.com
Weight Definition in Science Weights Definition Chemistry For example, water (h 2 o) has a formula weight of:. Molecular weight is used in chemistry to determine stoichiometry in chemical. Since 1961 the standard unit. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Molecular weight is a measure of the sum of the atomic weight values. Weights Definition Chemistry.
From gamesmartz.com
Weight Easy to Understand Weights Definition Chemistry Molecular weight is used in chemistry to determine stoichiometry in chemical. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Since 1961 the standard unit. 121 rows atomic weight, ratio. Weights Definition Chemistry.
From www.slideserve.com
PPT MASS (WEIGHT) & BALANCE PowerPoint Presentation ID2377094 Weights Definition Chemistry Their values are in the table of atomic weights,. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. The relative masses of the atoms are usually referred to as atomic weights. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element,. Weights Definition Chemistry.
From examsector.com
Atomic weight Definition, Units, & Table ExamSector Weights Definition Chemistry Molecular weight is used in chemistry to determine stoichiometry in chemical. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. The molecular weight is the total number of protons. Weights Definition Chemistry.
From www.wikihow.com
How to Calculate Molecular Weight 6 Steps (with Pictures) Weights Definition Chemistry Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Since 1961 the standard unit. In other words, it is the sum of the atomic masses of. Their values are in the table of atomic weights,. The relative masses of. Weights Definition Chemistry.
From primaryscienceonline.org.uk
Mass and Weight Primary Science Online Weights Definition Chemistry Since 1961 the standard unit. The relative masses of the atoms are usually referred to as atomic weights. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. 121 rows atomic weight, ratio of the average mass of a chemical. Weights Definition Chemistry.
From www.ssi.shimadzu.com
Molecular Weight Shimadzu Scientific Instruments Weights Definition Chemistry For example, water (h 2 o) has a formula weight of:. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Since 1961 the standard unit. The molecular weight is the total number of protons and neutrons in a compound. In other words, it is the sum of the atomic masses of. Atomic. Weights Definition Chemistry.
From www.geeksforgeeks.org
What is Mass & Weight? Definition, Differences, Examples & FAQs Weights Definition Chemistry Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Their values are in the table of atomic weights,. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The relative masses of the atoms are usually referred to as atomic weights. Atomic. Weights Definition Chemistry.
From www.slideserve.com
PPT PHYSICOCHEMICAL PRINCIPLES PowerPoint Presentation ID6847292 Weights Definition Chemistry Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The relative masses of. Weights Definition Chemistry.
From www.thoughtco.com
Difference Between Atomic Weight and Atomic Mass Weights Definition Chemistry Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. The molecular weight is the total number of protons and neutrons in a compound. Since 1961 the standard unit. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. In. Weights Definition Chemistry.
From www.wisc-online.com
Calculating Formula Weight and Molecular Weight OER Weights Definition Chemistry The molecular weight is the total number of protons and neutrons in a compound. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Their values are in the table of atomic weights,. Atomic weight and molecular weight are molar. Weights Definition Chemistry.
From blog.orendatech.com
Understanding the Mole Molecular Weights in Chemistry Weights Definition Chemistry The molecular weight is the total number of protons and neutrons in a compound. Their values are in the table of atomic weights,. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula.. Weights Definition Chemistry.
From in.pinterest.com
Difference Between Mass and Weight Physics lessons, Chemistry lessons Weights Definition Chemistry The relative masses of the atoms are usually referred to as atomic weights. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Since 1961 the standard unit. Molecular weight is a measure. Weights Definition Chemistry.
From www.slideserve.com
PPT The Atom & the Periodic Table PowerPoint Presentation, free Weights Definition Chemistry The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. In other words, it is the sum of the atomic masses of. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. 121 rows atomic weight, ratio of the average. Weights Definition Chemistry.
From www.youtube.com
What is Weight? YouTube Weights Definition Chemistry Molecular weight is used in chemistry to determine stoichiometry in chemical. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Their values are in the table of atomic weights,. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using. Weights Definition Chemistry.
From www.youtube.com
Atomic Weight and Average Atomic Mass Chemistry Tutorial YouTube Weights Definition Chemistry Their values are in the table of atomic weights,. Since 1961 the standard unit. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. The molecular weight is the total number of protons and neutrons in a compound. Atomic weight and molecular weight are molar quantities that relate to the. Weights Definition Chemistry.
From www.thoughtco.com
What Is the Difference Between Weight and Mass? Weights Definition Chemistry Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. For example, water (h 2 o) has a formula weight of:. Molecular weight is used in chemistry to determine stoichiometry in chemical. The molecular weight is the total number of protons and neutrons in a compound. Since 1961 the standard unit.. Weights Definition Chemistry.
From ck12.org
Selection of Appropriate Weight or Capacity Units CK12 Foundation Weights Definition Chemistry Their values are in the table of atomic weights,. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. For example, water (h 2 o) has a formula weight of:. The relative masses. Weights Definition Chemistry.
From www.slideserve.com
PPT Chem 1151 Ch. 2 PowerPoint Presentation, free download ID3579589 Weights Definition Chemistry The molecular weight is the total number of protons and neutrons in a compound. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. For example, water (h 2 o) has a formula weight of:. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to. Weights Definition Chemistry.
From www.thoughtco.com
What Is Molecular Weight? Chemistry Definition Weights Definition Chemistry In other words, it is the sum of the atomic masses of. The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Their values are in the table of atomic weights,. The relative masses of the atoms are usually referred to as atomic weights. 121 rows atomic weight, ratio of. Weights Definition Chemistry.
From insidechem.blogspot.com
Mass and Its Measurement INSIDE CHEMISTRY Weights Definition Chemistry For example, water (h 2 o) has a formula weight of:. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. In other words, it is the sum of the atomic masses of. The molecular weight is the total number. Weights Definition Chemistry.
From www.dreamstime.com
Metal Weights To Determine Weight. Calibration Weights. Calibration Weights Definition Chemistry In other words, it is the sum of the atomic masses of. Since 1961 the standard unit. Their values are in the table of atomic weights,. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. For example, water (h 2 o) has a formula weight of:. Atomic mass, which is. Weights Definition Chemistry.
From www.slideserve.com
PPT Atomic Weights PowerPoint Presentation, free download ID3197304 Weights Definition Chemistry Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The relative masses of the atoms are usually referred to as atomic weights. In other words, it is the sum of the atomic masses. Weights Definition Chemistry.
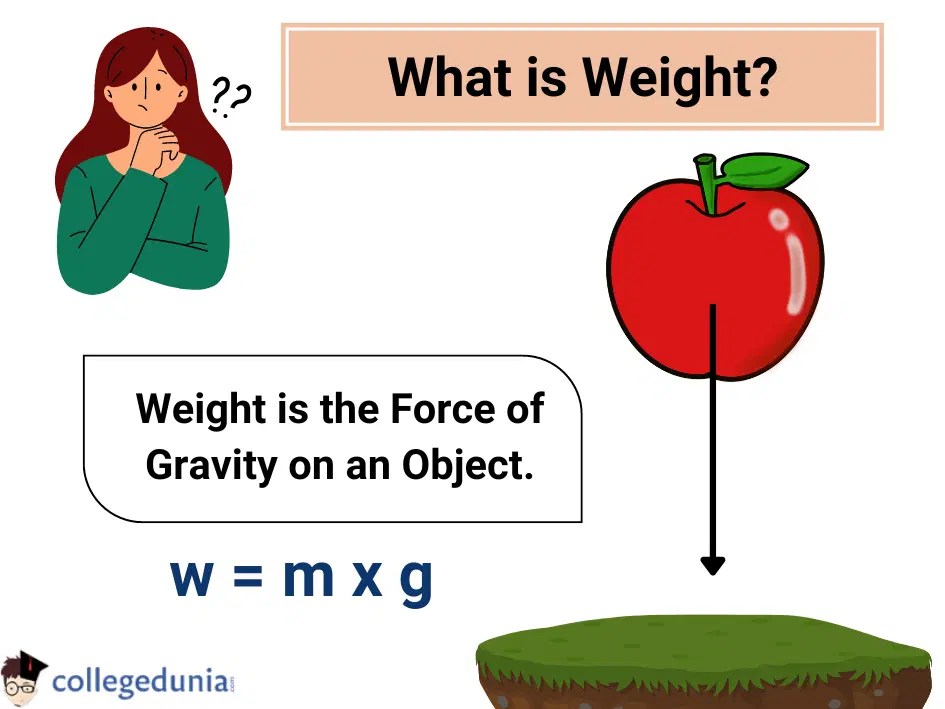
From collegedunia.com
Weight Formula Definition, Mass & Solved Examples Weights Definition Chemistry The relative masses of the atoms are usually referred to as atomic weights. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. The molecular weight is the total number of protons and neutrons in a compound. Atomic mass, which is also known as atomic weight, is the average mass of. Weights Definition Chemistry.
From cefyxppp.blob.core.windows.net
Weights Definition Oxford at Jeremy Olivarez blog Weights Definition Chemistry Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. The relative masses of the atoms are usually referred to as atomic weights. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Molecular weight is used in chemistry to determine. Weights Definition Chemistry.
From study.com
Equivalent Weight Overview & Formula How to Calculate Equivalent Weights Definition Chemistry 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Molecular weight is used in chemistry to determine stoichiometry in chemical. Since 1961 the standard unit. Their values are in the table of atomic weights,. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element,. Weights Definition Chemistry.
From www.slideserve.com
PPT Weight, Mass, Volume and Density PowerPoint Presentation, free Weights Definition Chemistry The relative masses of the atoms are usually referred to as atomic weights. The molecular weight is the total number of protons and neutrons in a compound. In other words, it is the sum of the atomic masses of. Their values are in the table of atomic weights,. The formula weight of a substance is the sum of the atomic. Weights Definition Chemistry.
From collegedunia.com
Weight Formula Definition, Mass & Solved Examples Weights Definition Chemistry In other words, it is the sum of the atomic masses of. 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. The molecular weight is the total number of protons and neutrons in a compound. Molecular weight is a measure of the sum of the atomic weight values of the atoms in. Weights Definition Chemistry.
From www.youtube.com
Atomic weights, Molecular weights and Formula weights Chemistry Weights Definition Chemistry The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Atomic weight and molecular weight are molar quantities that relate to the mass of an element or a compound, respectively. Since 1961 the standard unit. Molecular weight is used in chemistry to determine stoichiometry in chemical. The molecular weight is. Weights Definition Chemistry.
From www.tutorix.com
Difference Between Mass and Weight Weights Definition Chemistry Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Atomic weight and molecular weight are molar quantities that relate to. Weights Definition Chemistry.
From www.slideserve.com
PPT Atomic Weights PowerPoint Presentation, free download ID6807014 Weights Definition Chemistry The relative masses of the atoms are usually referred to as atomic weights. Molecular weight is used in chemistry to determine stoichiometry in chemical. Molecular weight is a measure of the sum of the atomic weight values of the atoms in a molecule. Since 1961 the standard unit. The formula weight of a substance is the sum of the atomic. Weights Definition Chemistry.
From sciencenotes.org
Mass vs Weight The Difference Between Mass and Weight Weights Definition Chemistry Molecular weight is used in chemistry to determine stoichiometry in chemical. The relative masses of the atoms are usually referred to as atomic weights. Their values are in the table of atomic weights,. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a. Weights Definition Chemistry.
From www.slideserve.com
PPT Physical and Chemical Properties PowerPoint Presentation, free Weights Definition Chemistry In other words, it is the sum of the atomic masses of. Molecular weight is used in chemistry to determine stoichiometry in chemical. The molecular weight is the total number of protons and neutrons in a compound. Their values are in the table of atomic weights,. Molecular weight is a measure of the sum of the atomic weight values of. Weights Definition Chemistry.
From www.youtube.com
Atomic weight Meaning YouTube Weights Definition Chemistry 121 rows atomic weight, ratio of the average mass of a chemical element’s atoms to some standard. Atomic mass, which is also known as atomic weight, is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally occurring element. For example, water (h 2 o) has a formula weight of:. The molecular. Weights Definition Chemistry.