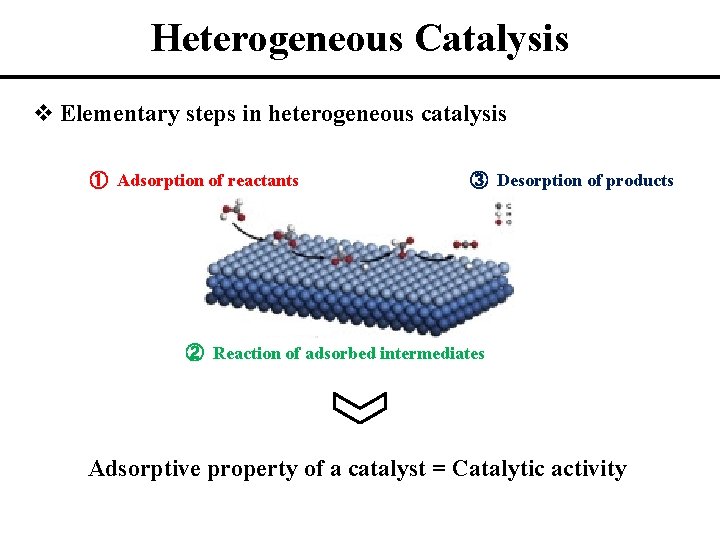
A Heterogeneous Catalyst Increases The Rate Of A Reaction By . a heterogeneous catalyst is present in a different phase from the reactants. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. At least one of the. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. Such catalysts are usually solids, and often function by furnishing an active.
from slidetodoc.com
In heterogeneous catalysis, the catalyst is in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active. At least one of the. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. a heterogeneous catalyst is present in a different phase from the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous.
Heterogeneous Catalysis Solid State Physics 141 A Dohyung
A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. a heterogeneous catalyst is present in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. At least one of the. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants.
From www.sasadoctor.com
Rate of reaction chemistry igcse A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung A Heterogeneous Catalyst Increases The Rate Of A Reaction By because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. Such catalysts are usually solids, and often function by furnishing an active. At least one of the. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. . A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From slideplayer.com
Catalysis A catalyst is a substance that increases the rate of a reaction without being consumed A Heterogeneous Catalyst Increases The Rate Of A Reaction By catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants.. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. this page looks. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Chapter 17 Equilibrium PowerPoint Presentation, free download ID1386098 A Heterogeneous Catalyst Increases The Rate Of A Reaction By Such catalysts are usually solids, and often function by furnishing an active. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. because a catalyst decreases the height of the energy barrier,. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.numerade.com
SOLVEDDoes a catalyst increase reaction rate by the same means as a rise in temperature does A Heterogeneous Catalyst Increases The Rate Of A Reaction By because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. this. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is present in a different phase from the reactants. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. . A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.ck12.org
Reaction Rate ( Video ) Chemistry CK12 Foundation A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. figure 1.1.1. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From slideplayer.com
Catalysis A catalyst is a substance that increases the rate of a reaction without being consumed A Heterogeneous Catalyst Increases The Rate Of A Reaction By In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. At least one. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) A Heterogeneous Catalyst Increases The Rate Of A Reaction By because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is present in a different phase from the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Presentation ID2087208 A Heterogeneous Catalyst Increases The Rate Of A Reaction By catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. Such catalysts are usually solids, and often function by furnishing an active. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is present in a different phase from. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From studyfullfarrior.z19.web.core.windows.net
Rates Of Reaction And Energy Changes A Heterogeneous Catalyst Increases The Rate Of A Reaction By catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Presentation ID3058051 A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Such catalysts are usually solids, and often function by furnishing an active. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. a heterogeneous catalyst is present in a. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. Such catalysts are usually solids, and often. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From byjus.com
How does a catalyst increase the rate of a reaction? A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is present in a different phase from the reactants. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. Such catalysts are. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 A Heterogeneous Catalyst Increases The Rate Of A Reaction By Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. figure 1.1.1 shows a general. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.numerade.com
SOLVED Question 1 2 pts Which types of catalysis can increase the rate of a chemical reaction A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. Such catalysts are usually solids, and often function by. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is present in a different phase from. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Heterogeneous catalysis PowerPoint Presentation, free download ID1984000 A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. catalysis (/. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From slideplayer.com
Catalysis A catalyst is a substance that increases the rate of a reaction without being consumed A Heterogeneous Catalyst Increases The Rate Of A Reaction By because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. a heterogeneous catalyst is present in a different phase from the reactants. Such catalysts are usually solids, and often function by. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.doubtnut.com
A catalyst increases rate of reaction by A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Such catalysts are usually solids, and often function by furnishing an active. At least one of the. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. catalysis (/ kəˈtæləsɪs /) is. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions And Equilibrium MCAT A Heterogeneous Catalyst Increases The Rate Of A Reaction By At least one of the. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is present. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.numerade.com
SOLVEDA catalyst increases rate of reaction by (a) decreasing enthalpy (b) decreasing A Heterogeneous Catalyst Increases The Rate Of A Reaction By catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Such catalysts are usually solids, and often function by furnishing an active.. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From study.com
Effect of Catalysts on Rates of Reaction Video & Lesson Transcript A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. Such catalysts are usually solids, and often function by furnishing an active.. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Gina blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is present in a different phase from the reactants. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. Such catalysts are usually solids, and often function by. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. At least one of the. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science A Heterogeneous Catalyst Increases The Rate Of A Reaction By In heterogeneous catalysis, the catalyst is in a different phase from the reactants. At least one of the. Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. because a catalyst decreases the height of the energy. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.researchgate.net
Diagram of catalytic heterogeneous reaction mechanism [88] Download Scientific Diagram A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. a heterogeneous catalyst is present in a different phase from the reactants. a heterogeneous catalyst is a catalyst. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideshare.net
Rate equations for heterogeneous reactions combining linear and nonli… A Heterogeneous Catalyst Increases The Rate Of A Reaction By Such catalysts are usually solids, and often function by furnishing an active. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. At least one of the. because a catalyst decreases the. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. a heterogeneous catalyst is present in a different phase from the reactants. At least one of the. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.savemyexams.com
Energy Profiles With & Without Catalysts HL IB Chemistry Revision Notes 2025 A Heterogeneous Catalyst Increases The Rate Of A Reaction By In heterogeneous catalysis, the catalyst is in a different phase from the reactants. a heterogeneous catalyst is a catalyst that is present in a different phase (usually a solid) than the reactants. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a catalyst[1][2] (/. figure 1.1.1. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From exoqpbamm.blob.core.windows.net
A Catalyst Increases The Rate Of A Reaction By Providing A Path That at Gina blog A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. this page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they. Such catalysts are usually solids, and often function by furnishing an active. a heterogeneous catalyst is present in a. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From slidetodoc.com
Heterogeneous Catalysis Solid State Physics 141 A Dohyung A Heterogeneous Catalyst Increases The Rate Of A Reaction By Such catalysts are usually solids, and often function by furnishing an active. In heterogeneous catalysis, the catalyst is in a different phase from the reactants. figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. At least one of the. a heterogeneous catalyst is present in a different phase from the reactants. . A Heterogeneous Catalyst Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts PowerPoint A Heterogeneous Catalyst Increases The Rate Of A Reaction By figure 1.1.1 shows a general effect of temperature on the reaction rate for a heterogeneous. because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and. catalysis (/ kəˈtæləsɪs /) is the increase in rate of a chemical reaction due to an added substance known as a. A Heterogeneous Catalyst Increases The Rate Of A Reaction By.