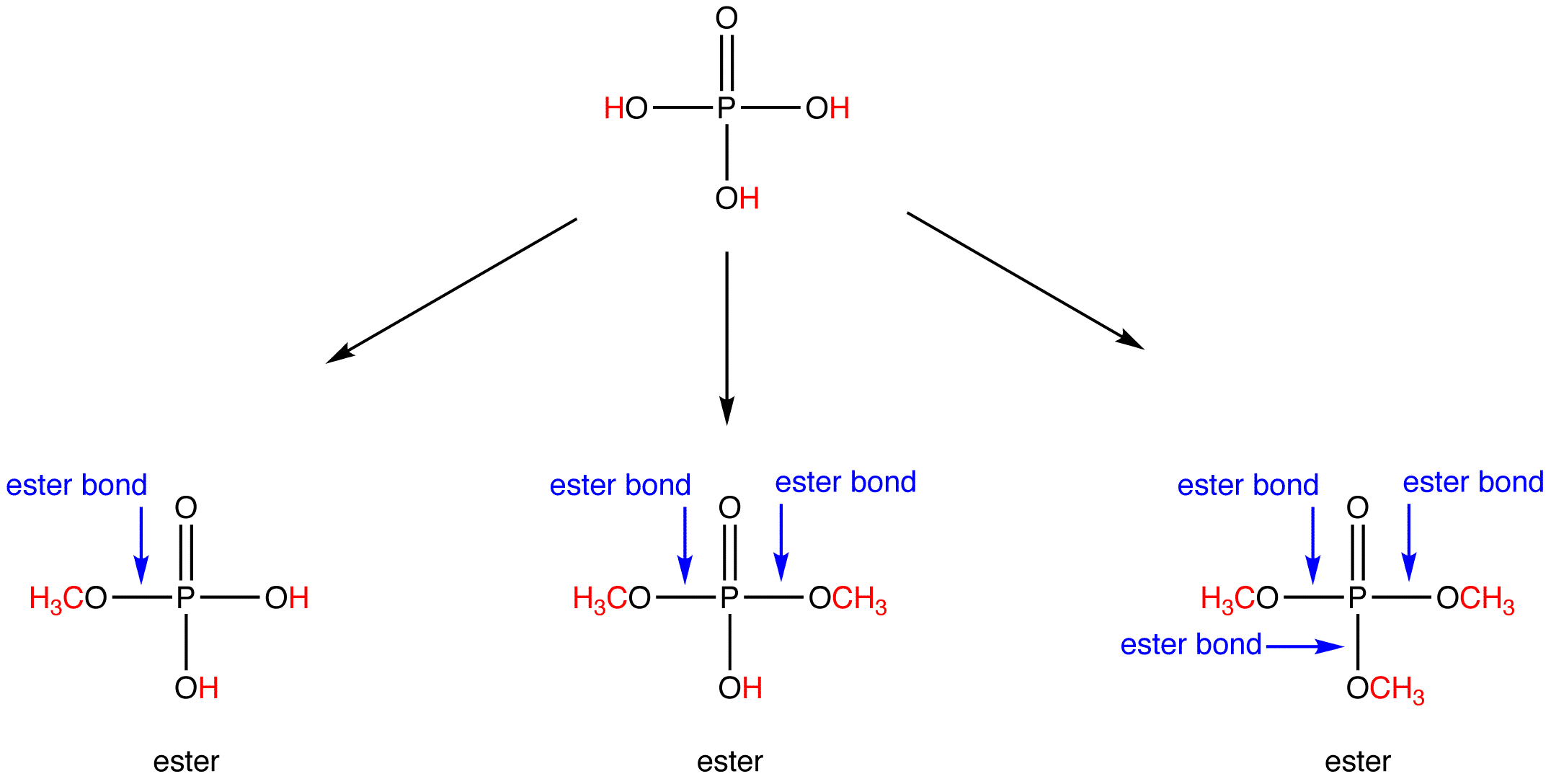
Are Ester Linkages Covalent . An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. For example, methyl butanoate is. For example, methyl butanoate is found in pineapple oil, and isopentyl. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. This reaction results in the formation of an.
from chem.libretexts.org
An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is found in pineapple oil, and isopentyl. For example, methyl butanoate is. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. This reaction results in the formation of an. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester.
Ester Chemistry LibreTexts
Are Ester Linkages Covalent This reaction results in the formation of an. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is found in pineapple oil, and isopentyl. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. This reaction results in the formation of an. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. For example, methyl butanoate is.
From chem.libretexts.org
12.4 Esters Chemistry LibreTexts Are Ester Linkages Covalent Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. This reaction results in the formation of an. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. For example, methyl butanoate is found. Are Ester Linkages Covalent.
From studymind.co.uk
Ester Bonding (ALevel Biology) Study Mind Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. An ester linkage. Are Ester Linkages Covalent.
From chem.libretexts.org
Ester Chemistry LibreTexts Are Ester Linkages Covalent An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in. Are Ester Linkages Covalent.
From www.chemistryviews.org
Covalent Organic Frameworks with Ester Links ChemistryViews Are Ester Linkages Covalent Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. This reaction results in the formation of an. Ester linkages are the fundamental structural components. Are Ester Linkages Covalent.
From www.slideserve.com
PPT Drug metabolism PowerPoint Presentation, free download ID2312754 Are Ester Linkages Covalent For example, methyl butanoate is. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Although esters are covalent compounds and salts are ionic, esters. Are Ester Linkages Covalent.
From www.biologyonline.com
Phosphodiester bond Definition and Examples Biology Online Dictionary Are Ester Linkages Covalent An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Discuss the wide occurrence of esters. Are Ester Linkages Covalent.
From www.animalia-life.club
Ester Bond Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. This reaction results in the. Are Ester Linkages Covalent.
From www.pinterest.fr
the diagram shows different types of hydrogens and their interactions Are Ester Linkages Covalent For example, methyl butanoate is. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. An ester linkage is a type of covalent bond formed. Are Ester Linkages Covalent.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples Are Ester Linkages Covalent An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is. This reaction results in the formation of an. The process, known as esterification, joins the acid. Are Ester Linkages Covalent.
From chem.libretexts.org
21.6 Chemistry of Esters Chemistry LibreTexts Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. For example, methyl butanoate is. This reaction results in the formation of an. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Discuss the wide occurrence of esters in nature, and their. Are Ester Linkages Covalent.
From www.researchgate.net
Intramolecular covalent linkages in Grampositive pili and MSCRAMMs Are Ester Linkages Covalent Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. The process, known as esterification, joins the acid. Are Ester Linkages Covalent.
From byjus.com
19. IN both DNA RNA , heterocyclic base phosphate ester linkages are at Are Ester Linkages Covalent Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. Although esters are covalent compounds and salts are ionic, esters are named. Are Ester Linkages Covalent.
From wou.edu
CH103 Chapter 8 The Major Macromolecules Chemistry Are Ester Linkages Covalent For example, methyl butanoate is. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. This reaction results in the formation of an. For example, methyl butanoate is found in pineapple oil, and isopentyl. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example. Are Ester Linkages Covalent.
From www.biologyonline.com
Phosphodiester bond Definition and Examples Biology Online Dictionary Are Ester Linkages Covalent The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example. Are Ester Linkages Covalent.
From www.slideshare.net
DNA structure Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. For example, methyl butanoate is found in pineapple oil, and isopentyl. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Write an equation to describe the hydrolysis of an ester under acidic. Are Ester Linkages Covalent.
From study.com
What is an Ester Linkage? Video & Lesson Transcript Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. For example, methyl butanoate is. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. This reaction results in the formation of an. The process, known as esterification, joins the acid and alcohol together by an ester bond (or. Are Ester Linkages Covalent.
From socratic.org
What is ester linkage in lipids? Socratic Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. This reaction results in the formation of an. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one. Are Ester Linkages Covalent.
From chem.libretexts.org
Ester Chemistry LibreTexts Are Ester Linkages Covalent Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Although esters are covalent compounds and salts are ionic, esters are named in a. Are Ester Linkages Covalent.
From www.researchgate.net
(A) Formation and disintegration of dynamiccovalent nanoaggregates via Are Ester Linkages Covalent An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Write an equation to describe the. Are Ester Linkages Covalent.
From www.researchgate.net
a) Comparison of imines and boronate esters as privileged linkages in Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. For example, methyl butanoate is. An ester linkage is. Are Ester Linkages Covalent.
From www.chemistrysteps.com
Naming Esters Chemistry Steps Are Ester Linkages Covalent Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example. Are Ester Linkages Covalent.
From www.chegg.com
Solved Ester linkages are widely used in nature to make Are Ester Linkages Covalent Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. For example, methyl butanoate is found in pineapple oil, and isopentyl. This. Are Ester Linkages Covalent.
From www.slideserve.com
PPT Molecular mechanisms of DNA replication. Transcription Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. This reaction results in the formation of an. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is. The process, known as esterification, joins the acid and alcohol. Are Ester Linkages Covalent.
From pubs.acs.org
Chemical Conversion of Linkages in Covalent Organic Frameworks Are Ester Linkages Covalent The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. For example, methyl butanoate is. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. This reaction results in the. Are Ester Linkages Covalent.
From www.researchgate.net
Mechanisms for formation of covalent bonds a EDAC mediated amide bond Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. This reaction results in the formation of an. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions.. Are Ester Linkages Covalent.
From www.researchgate.net
Condensations of widely used types of linkages in COFs (a) boroxine Are Ester Linkages Covalent For example, methyl butanoate is. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. For example, methyl butanoate is found in pineapple oil,. Are Ester Linkages Covalent.
From www.biologyonline.com
Crosslinking Definition and Examples Biology Online Dictionary Are Ester Linkages Covalent For example, methyl butanoate is. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. For example, methyl butanoate. Are Ester Linkages Covalent.
From www.chemistrysteps.com
Amides Structure and Reactivity Chemistry Steps Are Ester Linkages Covalent Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. For example, methyl butanoate is. This reaction results in the formation of an. Ester linkages are the fundamental structural components that give waxes, fats,. Are Ester Linkages Covalent.
From www.guidechem.com
In the DNA backbone, which bonds exactly are considered ester bonds Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is. An ester linkage. Are Ester Linkages Covalent.
From www.youtube.com
Ester Linkage, Chemistry Lecture Sabaq.pk YouTube Are Ester Linkages Covalent This reaction results in the formation of an. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Discuss. Are Ester Linkages Covalent.
From www.chemistrystudent.com
Esters (ALevel) ChemistryStudent Are Ester Linkages Covalent Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. An ester linkage. Are Ester Linkages Covalent.
From www.pinterest.com
Ester vs. Ether Linkages & Isoprenes Organic chemistry, Chemistry, Ester Are Ester Linkages Covalent Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. For example, methyl butanoate is found in pineapple oil, and isopentyl. This reaction results in the formation of an. Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of. Are Ester Linkages Covalent.
From www.animalia-life.club
Ester Functional Group Examples Are Ester Linkages Covalent Write an equation to describe the hydrolysis of an ester under acidic or basic conditions. Ester linkages are the fundamental structural components that give waxes, fats, and oils their unique properties. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. The process, known as esterification, joins the acid and alcohol together. Are Ester Linkages Covalent.
From onlinelibrary.wiley.com
Quantitative Construction of Boronic‐Ester Linkages in Covalent Organic Are Ester Linkages Covalent Discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester. The process, known as esterification, joins the acid and alcohol together by an ester bond (or ester linkage) and creates. An ester linkage is a type of covalent bond formed. Are Ester Linkages Covalent.
From www.researchgate.net
Typical covalent linkages employed in construction of COFs. Download Are Ester Linkages Covalent Although esters are covalent compounds and salts are ionic, esters are named in a manner similar to that used for naming. For example, methyl butanoate is found in pineapple oil, and isopentyl. This reaction results in the formation of an. An ester linkage is a type of covalent bond formed when a carboxylic acid reacts with an alcohol. For example,. Are Ester Linkages Covalent.