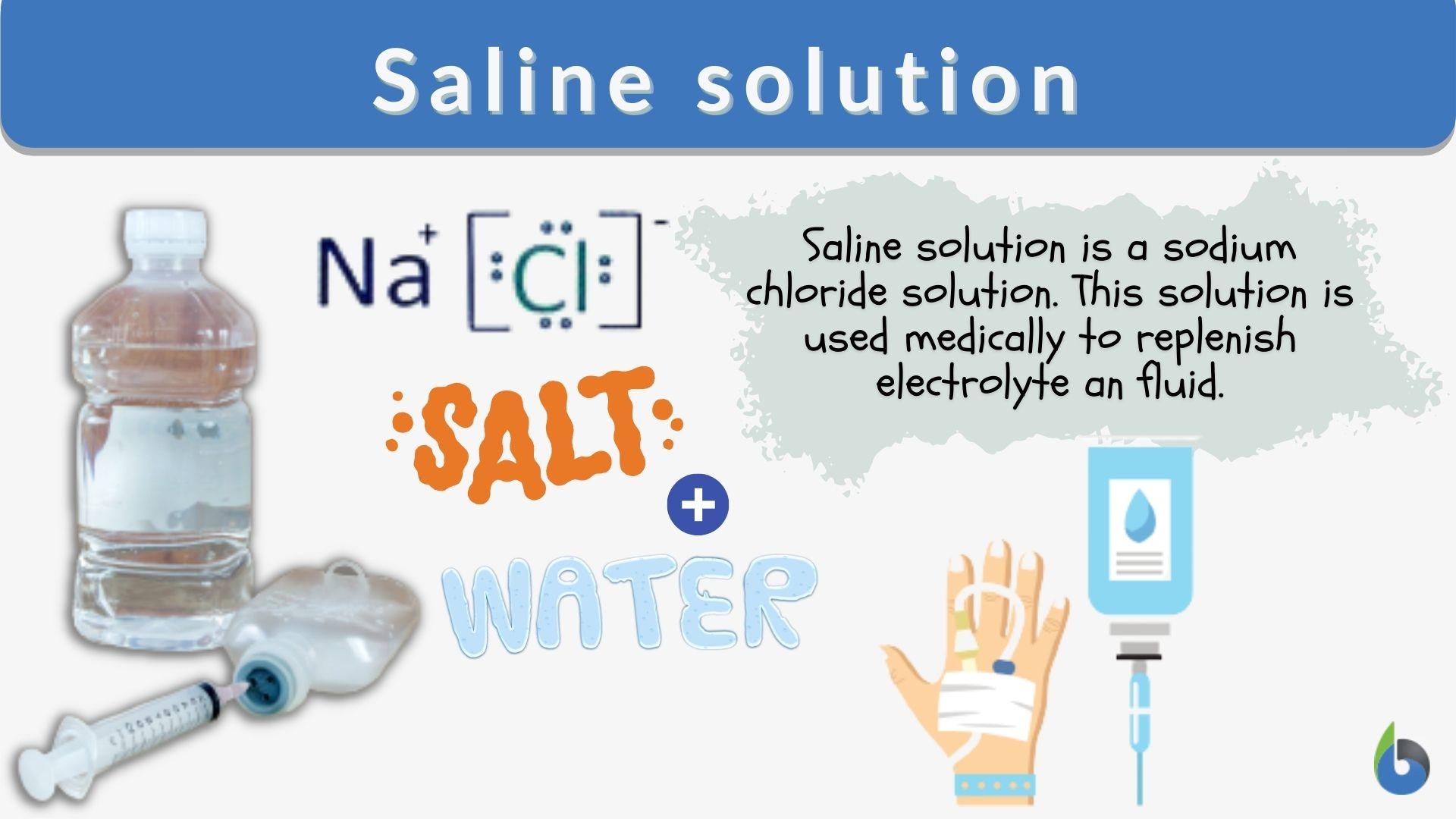
How To Make Saline Solution In Laboratory . Recipe can be automatically scaled by entering desired final. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. The solution can be used as a disinfectant or sterile rinse or for lab work. Calculate the mass of sodium chloride needed to prepare 250. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. Normal saline, a 0.9% sodium chloride. The solution can be used to disinfect, sterilize, or do laboratory work. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. A saline solution’s salt hinders bacterial growth while washing away pollutants. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water.
from www.biologyonline.com
This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. The solution can be used to disinfect, sterilize, or do laboratory work. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Normal saline, a 0.9% sodium chloride. Recipe can be automatically scaled by entering desired final. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water.
Saline solution Definition and Examples Biology Online Dictionary
How To Make Saline Solution In Laboratory Calculate the mass of sodium chloride needed to prepare 250. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. Recipe can be automatically scaled by entering desired final. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. A saline solution’s salt hinders bacterial growth while washing away pollutants. The solution can be used as a disinfectant or sterile rinse or for lab work. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. The solution can be used to disinfect, sterilize, or do laboratory work. Calculate the mass of sodium chloride needed to prepare 250. Normal saline, a 0.9% sodium chloride. The salt in a saline solution discourages bacterial growth while rinsing away contaminants.
From www.warbyparker.com
What Is Saline Solution? Warby Parker How To Make Saline Solution In Laboratory Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. Recipe can be automatically scaled by entering desired final. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of. How To Make Saline Solution In Laboratory.
From www.wikihow.com
How to Make a Saline Solution 8 Steps (with Pictures) wikiHow How To Make Saline Solution In Laboratory A saline solution’s salt hinders bacterial growth while washing away pollutants. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. The normal saline solution is. How To Make Saline Solution In Laboratory.
From www.supsalv.org
How to Make Saline Solution A Beginner’s Guide to Natural Relief The How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. A saline solution’s salt hinders bacterial growth while washing away pollutants. Calculate the mass of sodium chloride needed to prepare 250. This recipe is for a salt solution that is normal, meaning it. How To Make Saline Solution In Laboratory.
From retakeagain.blogspot.com
How To Make Normal Saline Solution At Home Retake Again How To Make Saline Solution In Laboratory Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. Normal saline, a 0.9% sodium chloride. The solution can be used as a disinfectant or sterile rinse or for lab work. The solution can be used to disinfect, sterilize, or do laboratory work. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to,. How To Make Saline Solution In Laboratory.
From www.drshellenberger.com
How to Make a Nasal Saline Solution — Dr. Luke Shellenberger How To Make Saline Solution In Laboratory Normal saline, a 0.9% sodium chloride. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. The solution can be used to disinfect, sterilize, or do laboratory work. This recipe is for. How To Make Saline Solution In Laboratory.
From www.medicalnewstoday.com
How to make saline solution at home Ingredients and uses How To Make Saline Solution In Laboratory Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. The solution can be used as a disinfectant or sterile rinse or for lab work. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled. How To Make Saline Solution In Laboratory.
From www.pinterest.com
How To Make (And Use) Saline Solution To Stay Healthy Health, Saline How To Make Saline Solution In Laboratory Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Recipe can be automatically scaled by entering desired final. Pbs (phosphate buffered saline) (1x,. How To Make Saline Solution In Laboratory.
From supermamiwannabe-english.blogspot.com
How to make your own saline solution at home How To Make Saline Solution In Laboratory The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Pbs (phosphate buffered saline). How To Make Saline Solution In Laboratory.
From www.branchor.com
How to Make Saline Solution The Ultimate Guide The Explanation Express How To Make Saline Solution In Laboratory Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. A saline solution’s salt hinders bacterial growth while washing away pollutants. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. This recipe makes a normal salt. How To Make Saline Solution In Laboratory.
From www.youtube.com
Preparation of Normal saline0.85 NaCl YouTube How To Make Saline Solution In Laboratory Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. The normal saline solution is simply. How To Make Saline Solution In Laboratory.
From www.biologyonline.com
Saline solution Definition and Examples Biology Online Dictionary How To Make Saline Solution In Laboratory Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. The salt in a saline solution. How To Make Saline Solution In Laboratory.
From www.pc-mobile.net
How to Make Saline Solution A StepbyStep Guide for Various Purposes How To Make Saline Solution In Laboratory This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. A saline solution’s salt hinders bacterial growth while washing away pollutants. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Normal saline (0.85 %) has been recommended by various manufacturers. How To Make Saline Solution In Laboratory.
From www.duhoctrungquoc.vn
How to Make a Saline Solution Wiki First Aid and Emergency Health How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. Normal saline, a 0.9% sodium. How To Make Saline Solution In Laboratory.
From www.amazon.com
Base Labs 7 Hypertonic Saline Solution for Nebulizer How To Make Saline Solution In Laboratory The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Calculate the mass of sodium chloride needed to prepare 250. The solution can be used to disinfect, sterilize, or do laboratory work. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in. How To Make Saline Solution In Laboratory.
From fr.wikihow.com
Comment préparer une solution saline 9 étapes How To Make Saline Solution In Laboratory This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. Recipe can be automatically scaled by entering desired final. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in. How To Make Saline Solution In Laboratory.
From www.wikihow.com
The 2 Best Ways to Make a Saline Solution wikiHow How To Make Saline Solution In Laboratory A saline solution’s salt hinders bacterial growth while washing away pollutants. Recipe can be automatically scaled by entering desired final. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Calculate the mass of sodium chloride needed to prepare 250. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution. How To Make Saline Solution In Laboratory.
From animalia-life.club
Normal Saline Solution How To Make Saline Solution In Laboratory The salt in a saline solution discourages bacterial growth while rinsing away contaminants. The solution can be used to disinfect, sterilize, or do laboratory work. A saline solution’s salt hinders bacterial growth while washing away pollutants. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Recipe can be automatically scaled by. How To Make Saline Solution In Laboratory.
From www.thoughtco.com
How to Make Saline Solution at Home How To Make Saline Solution In Laboratory Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. The solution can be used as a disinfectant or sterile rinse or for lab work. Normal saline, a 0.9% sodium chloride. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can. How To Make Saline Solution In Laboratory.
From www.happysimpleliving.com
Homemade Saline Solution Recipe Happy Simple Living How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Calculate the mass. How To Make Saline Solution In Laboratory.
From littlebinsforlittlehands.com
How To Make Saline Solution Slime Recipe for Kid's Science How To Make Saline Solution In Laboratory This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. A saline solution’s salt hinders bacterial growth while washing away pollutants. Recipe can be automatically scaled by entering desired final. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the. How To Make Saline Solution In Laboratory.
From www.youtube.com
How to Make a Saline Solution at Home YouTube How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. The solution can be used as a disinfectant or sterile rinse or for lab work. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. Calculate the mass of sodium chloride needed to prepare 250. The solution can be used to disinfect, sterilize, or do laboratory work. Another. How To Make Saline Solution In Laboratory.
From www.youtube.com
How to Make A Saline Solution For Your Nebulizer YouTube How To Make Saline Solution In Laboratory The salt in a saline solution discourages bacterial growth while rinsing away contaminants. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. A saline solution’s salt hinders bacterial growth while washing away pollutants. Recipe can be automatically scaled by entering desired final. Normal saline (0.85 %) has been recommended by. How To Make Saline Solution In Laboratory.
From retakeagain.blogspot.com
How To Make Normal Saline Solution At Home Retake Again How To Make Saline Solution In Laboratory The salt in a saline solution discourages bacterial growth while rinsing away contaminants. The solution can be used to disinfect, sterilize, or do laboratory work. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. A saline solution’s salt hinders bacterial growth while washing away pollutants. Pbs (phosphate buffered. How To Make Saline Solution In Laboratory.
From lessonlangdonslurp.z21.web.core.windows.net
Saline Solution Instrumental How To Make Saline Solution In Laboratory This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. Normal saline, a 0.9% sodium chloride. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing. How To Make Saline Solution In Laboratory.
From www.dreamstime.com
Experiment with Salt and Water. Making a Saline Water Solution Stock How To Make Saline Solution In Laboratory The solution can be used to disinfect, sterilize, or do laboratory work. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Recipe can be automatically scaled by entering desired final. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids.. How To Make Saline Solution In Laboratory.
From www.watercures.org
Saline IV Solution vs Water Cures Protocol The Salt Controversy? How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. The solution can be used as a disinfectant or sterile rinse or for lab work. Normal saline. How To Make Saline Solution In Laboratory.
From bigamart.com
Base Labs 7 Hypertonic Saline Solution for Nebulisers Sterile 7 How To Make Saline Solution In Laboratory This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Another solution commonly used for intravenous injections is normal saline, a 0.16 m solution of sodium chloride in water. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. Normal saline, a 0.9% sodium chloride. Recipe can be automatically scaled by. How To Make Saline Solution In Laboratory.
From babymommytime.com
Homemade Saline Solution Recipe For Nebulizer Babies & Toddlers How To Make Saline Solution In Laboratory This recipe is for a salt solution that is normal, meaning it is the same concentration as, or isotonic to, body fluids. Calculate the mass of sodium chloride needed to prepare 250. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. Normal saline, a 0.9% sodium chloride. The normal saline solution. How To Make Saline Solution In Laboratory.
From www.youtube.com
Saline Solution How to Make a Saline Solution YouTube How To Make Saline Solution In Laboratory The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. The salt in a saline solution discourages bacterial growth while rinsing away contaminants. Normal saline (0.85 %) has been recommended by various manufacturers and authors. How To Make Saline Solution In Laboratory.
From www.slideserve.com
PPT Make a Saline Solution at Home to Clean and Disinfect Wounds How To Make Saline Solution In Laboratory Recipe can be automatically scaled by entering desired final. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. The solution can be used as a disinfectant or sterile rinse or for lab work. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and.. How To Make Saline Solution In Laboratory.
From www.pinterest.com
How to Make a Homemade Normal Saline Solution Saline solution, Saline How To Make Saline Solution In Laboratory This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. A saline solution’s salt hinders bacterial growth while washing away pollutants. Normal saline (0.85 %) has been recommended by various manufacturers and authors for preparing test suspensions of organisms to help maintain bacterial cell integrity and. The salt in a saline solution. How To Make Saline Solution In Laboratory.
From solvedlib.com
Normal saline solution is 0.90 NaCl . How many gram… SolvedLib How To Make Saline Solution In Laboratory The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving. Calculate the mass of sodium chloride needed to prepare 250. Pbs (phosphate buffered saline) (1x, ph 7.4) preparation guide and recipe. The solution can be used as a disinfectant or sterile rinse or for lab work. The salt in. How To Make Saline Solution In Laboratory.
From bestdiypro.com
How to Make Saline Solution A Comprehensive Guide for Home Use Best How To Make Saline Solution In Laboratory Normal saline, a 0.9% sodium chloride. The solution can be used as a disinfectant or sterile rinse or for lab work. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. A saline solution’s salt. How To Make Saline Solution In Laboratory.
From wikihow.com
The 2 Best Ways to Make a Saline Solution wikiHow How To Make Saline Solution In Laboratory The solution can be used as a disinfectant or sterile rinse or for lab work. This recipe makes a normal salt solution, which is the same concentration as, or isotonic to, bodily fluids. The solution can be used to disinfect, sterilize, or do laboratory work. A saline solution’s salt hinders bacterial growth while washing away pollutants. The salt in a. How To Make Saline Solution In Laboratory.
From littlebinsforlittlehands.com
How To Make Saline Solution Slime Recipe for Kid's Science How To Make Saline Solution In Laboratory The salt in a saline solution discourages bacterial growth while rinsing away contaminants. The normal saline solution is simply the 0.85% sodium chloride (nacl) solution which can be prepared in the laboratory by dissolving the calculated amount of sodium chloride crystals in the required quantity of distilled water. Recipe can be automatically scaled by entering desired final. Normal saline (0.85. How To Make Saline Solution In Laboratory.