Lead Carbonate And Hydrochloric Acid . Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: During the process, red or brown intermediates can sometimes be observed: This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Titration must be used if the reactants are soluble. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Soluble salts can be made by reacting acids with soluble or insoluble reactants.
from www.chegg.com
This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It describes the formation of lead (ii). During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Soluble salts can be made by reacting acids with soluble or insoluble reactants. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Titration must be used if the reactants are soluble.
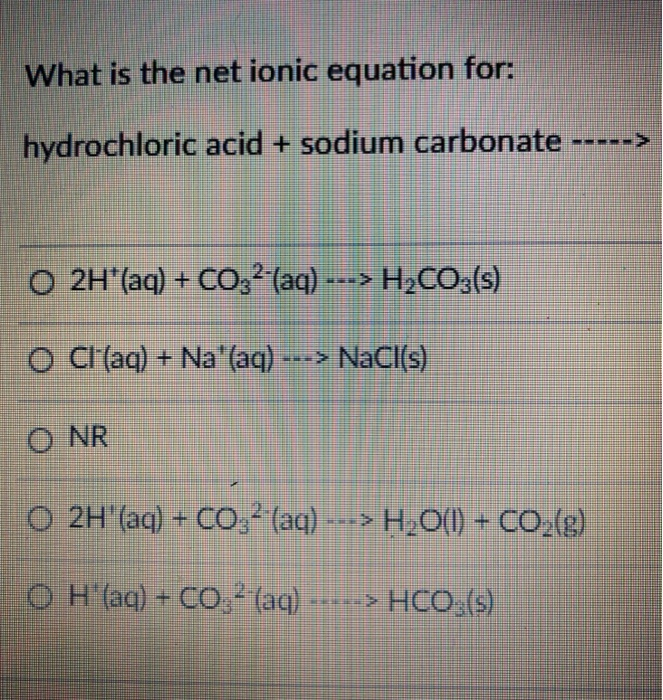
Solved What is the net ionic equation for hydrochloric acid
Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: During the process, red or brown intermediates can sometimes be observed: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. It describes the formation of lead (ii). When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water.
From wisc.pb.unizin.org
4.2 Classifying Chemical Reactions Chemistry Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Lead(ii) is precipitated by sulfide in. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
hydrochloric acid reacts with Sodium carbonate YouTube Lead Carbonate And Hydrochloric Acid This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Soluble salts can be made by reacting acids with soluble or insoluble. Lead Carbonate And Hydrochloric Acid.
From brainly.in
47. Explain the reaction of sodium carbonate with hydrochloric acid by Lead Carbonate And Hydrochloric Acid This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) +. Lead Carbonate And Hydrochloric Acid.
From www.alamy.com
Diagram of the laboratory preparation of carbon dioxide from Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. During the process, red or brown intermediates can sometimes be observed: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead(ii). Lead Carbonate And Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Observations of the Reaction between Lead Carbonate And Hydrochloric Acid Titration must be used if the reactants are soluble. It describes the formation of lead (ii). When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Soluble salts can be made by reacting acids with soluble or insoluble reactants. During the process, red or brown intermediates can sometimes. Lead Carbonate And Hydrochloric Acid.
From ppt-online.org
Rates of reaction презентация онлайн Lead Carbonate And Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l). Lead Carbonate And Hydrochloric Acid.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP The Periodic Table Lead Carbonate And Hydrochloric Acid This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. \[\ce{pb^{2+}(aq) + 2nh3(aq). Lead Carbonate And Hydrochloric Acid.
From www.sciencephoto.com
Magnesium carbonate in hydrochloric acid Stock Image A500/0601 Lead Carbonate And Hydrochloric Acid Titration must be used if the reactants are soluble. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. During the process, red or brown intermediates can sometimes be observed: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. When acids react with carbonates, such. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
How to Balance BaCO3 + HCl = BaCl2 + H2O + CO2 Barium carbonate Lead Carbonate And Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white. Lead Carbonate And Hydrochloric Acid.
From www.alamy.com
Calcium carbonate hydrochloric acid hires stock photography and images Lead Carbonate And Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. When acids react with carbonates, such as calcium carbonate (found in chalk,. Lead Carbonate And Hydrochloric Acid.
From www.vrogue.co
Calcium Carbonate Hydrochloric Acid The Periodic Tabl vrogue.co Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. It describes the formation of lead (ii). This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Soluble salts can be made by reacting acids. Lead Carbonate And Hydrochloric Acid.
From www.numerade.com
The carbonate of metal M has the formula MCO3. This metal carbonate Lead Carbonate And Hydrochloric Acid This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Titration must be used if the reactants are soluble. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page looks at. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
The Reaction Between Hydrochloric Acid and Sodium Carbonate YouTube Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. It describes the formation of lead (ii). Lead(ii) ion reacts with aqueous ammonia to. Lead Carbonate And Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Lead Carbonate And Hydrochloric Acid This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. During the process, red or brown intermediates can sometimes be observed: Titration must be used if the reactants are soluble. It describes the formation of lead (ii). \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Soluble salts can be made by reacting acids with. Lead Carbonate And Hydrochloric Acid.
From www.slideserve.com
PPT ACIDS & BASES PowerPoint Presentation, free download ID2732225 Lead Carbonate And Hydrochloric Acid It describes the formation of lead (ii). This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. This page looks at the reactions between. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. It describes the formation of lead (ii). This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Soluble salts can be made by reacting acids with soluble or insoluble reactants. During the process, red or brown intermediates can sometimes be observed: When acids react with carbonates,. Lead Carbonate And Hydrochloric Acid.
From www.slideserve.com
PPT Water The Universal Solvent PowerPoint Presentation, free Lead Carbonate And Hydrochloric Acid When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Titration must be used if the reactants are soluble. Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the. Lead Carbonate And Hydrochloric Acid.
From mavink.com
Calcium Carbonate And Dilute Hydrochloric Acid Lead Carbonate And Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: During the process, red or brown intermediates can sometimes be observed: Soluble salts can. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
Sodium carbonate and Hydrochloric acid (HCl) reaction !Chemistry Lead Carbonate And Hydrochloric Acid This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Titration must be used if the reactants are soluble. This page looks at the formation of some insoluble lead (ii) compounds. Lead Carbonate And Hydrochloric Acid.
From www.vrogue.co
Solved 9 Calcium Carbonate Reacts With Hydrochloric A vrogue.co Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. It describes the formation of lead (ii). This. Lead Carbonate And Hydrochloric Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Lead Carbonate And Hydrochloric Acid.
From www.numerade.com
SOLVED Write a net ionic equation for the reaction that occurs when Lead Carbonate And Hydrochloric Acid Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. It describes the formation of lead (ii). Soluble salts can be made by reacting acids with soluble or insoluble reactants. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. When acids react with carbonates, such as calcium carbonate (found. Lead Carbonate And Hydrochloric Acid.
From www.bartleby.com
Answered (d) ammonium carbonate and hydrochloric… bartleby Lead Carbonate And Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. During the process, red or brown intermediates can sometimes be observed: This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. It describes the formation of lead (ii). \[\ce{pb^{2+}(aq) + 2nh3(aq) +. Lead Carbonate And Hydrochloric Acid.
From www.slideshare.net
Reaction types Lead Carbonate And Hydrochloric Acid This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Titration must be. Lead Carbonate And Hydrochloric Acid.
From www.youtube.com
COPPER(II) CARBONATE & HYDROCHLORIC ACID DEMONSTRATION YouTube Lead Carbonate And Hydrochloric Acid Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Titration must be used if the reactants are soluble. This page looks at the reactions between acids and carbonates to give. Lead Carbonate And Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Lead Carbonate And Hydrochloric Acid It describes the formation of lead (ii). Titration must be used if the reactants are soluble. During the process, red or brown intermediates can sometimes be observed: This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions. Lead Carbonate And Hydrochloric Acid.
From studymoose.com
Carbonate and hydrochloric acid Free Essay Example Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. During the process, red or brown intermediates can sometimes be observed: Soluble salts can be. Lead Carbonate And Hydrochloric Acid.
From www.chegg.com
Solved 4. sodium carbonate (aq)+ hydrochloric acid (aq)→ Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt,. Lead Carbonate And Hydrochloric Acid.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction of Solid Lead Carbonate And Hydrochloric Acid Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. It describes the formation of lead (ii). This page looks. Lead Carbonate And Hydrochloric Acid.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Lead Carbonate And Hydrochloric Acid This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Titration must be used if the reactants are soluble. During the process, red or brown intermediates can sometimes be observed: Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: Lead(ii) is precipitated. Lead Carbonate And Hydrochloric Acid.
From www.tessshebaylo.com
Write The Balanced Chemical Equation For Reaction Of Sodium Carbonate Lead Carbonate And Hydrochloric Acid Titration must be used if the reactants are soluble. When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. This page looks at the formation of some insoluble lead (ii) compounds from aqueous lead (ii) ions using precipitation reactions. Soluble salts can be made by reacting acids with. Lead Carbonate And Hydrochloric Acid.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Lead Carbonate And Hydrochloric Acid This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. During the process, red or brown intermediates can sometimes be observed: This page looks at the formation of some insoluble lead (ii) compounds from aqueous. Lead Carbonate And Hydrochloric Acid.
From www.chegg.com
Solved What is the net ionic equation for hydrochloric acid Lead Carbonate And Hydrochloric Acid \[\ce{pb^{2+}(aq) + 2nh3(aq) + 3h2o(l) + 2no3^{. Titration must be used if the reactants are soluble. This page looks at the reactions between acids and carbonates to give a salt, carbon dioxide and water. Soluble salts can be made by reacting acids with soluble or insoluble reactants. This page looks at the formation of some insoluble lead (ii) compounds from. Lead Carbonate And Hydrochloric Acid.
From childhealthpolicy.vumc.org
💄 Calcium carbonate reacts with hydrochloric acid balanced equation Lead Carbonate And Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: When acids react with carbonates, such as calcium carbonate (found in chalk, limestone and marble), a salt, water and carbon dioxide are. Lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide: This page looks at the reactions between acids. Lead Carbonate And Hydrochloric Acid.
From www.vrogue.co
Calcium Carbonate And Hydrochloric Acid vrogue.co Lead Carbonate And Hydrochloric Acid Soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be used if the reactants are soluble. This page discusses the precipitation of insoluble lead (ii) compounds from aqueous lead (ii) ions in solution. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. During the process, red or brown intermediates can sometimes be observed:. Lead Carbonate And Hydrochloric Acid.