What Are The 2 Main Functions Of A Battery . We need to understand some fundamentals of how electricity works before we can understand the battery. This is known as electrochemistry and the system that underpins a battery is. When you put those batteries into the flashlight and. Batteries power our lives by transforming energy from one type to another. Two terminals made of different chemicals (typically metals), the anode and the. Firstly, electricity is the flow of. To accept and release energy, a battery is coupled to. A battery is a device that stores chemical energy, and converts it to electricity. Whether a traditional disposable battery (e.g., aa) or a rechargeable. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. There are three main components of a battery: To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight.
from www.thervgeeks.com
To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. Two terminals made of different chemicals (typically metals), the anode and the. A battery is a device that stores chemical energy, and converts it to electricity. To accept and release energy, a battery is coupled to. Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). We need to understand some fundamentals of how electricity works before we can understand the battery. When you put those batteries into the flashlight and. Firstly, electricity is the flow of. There are three main components of a battery:
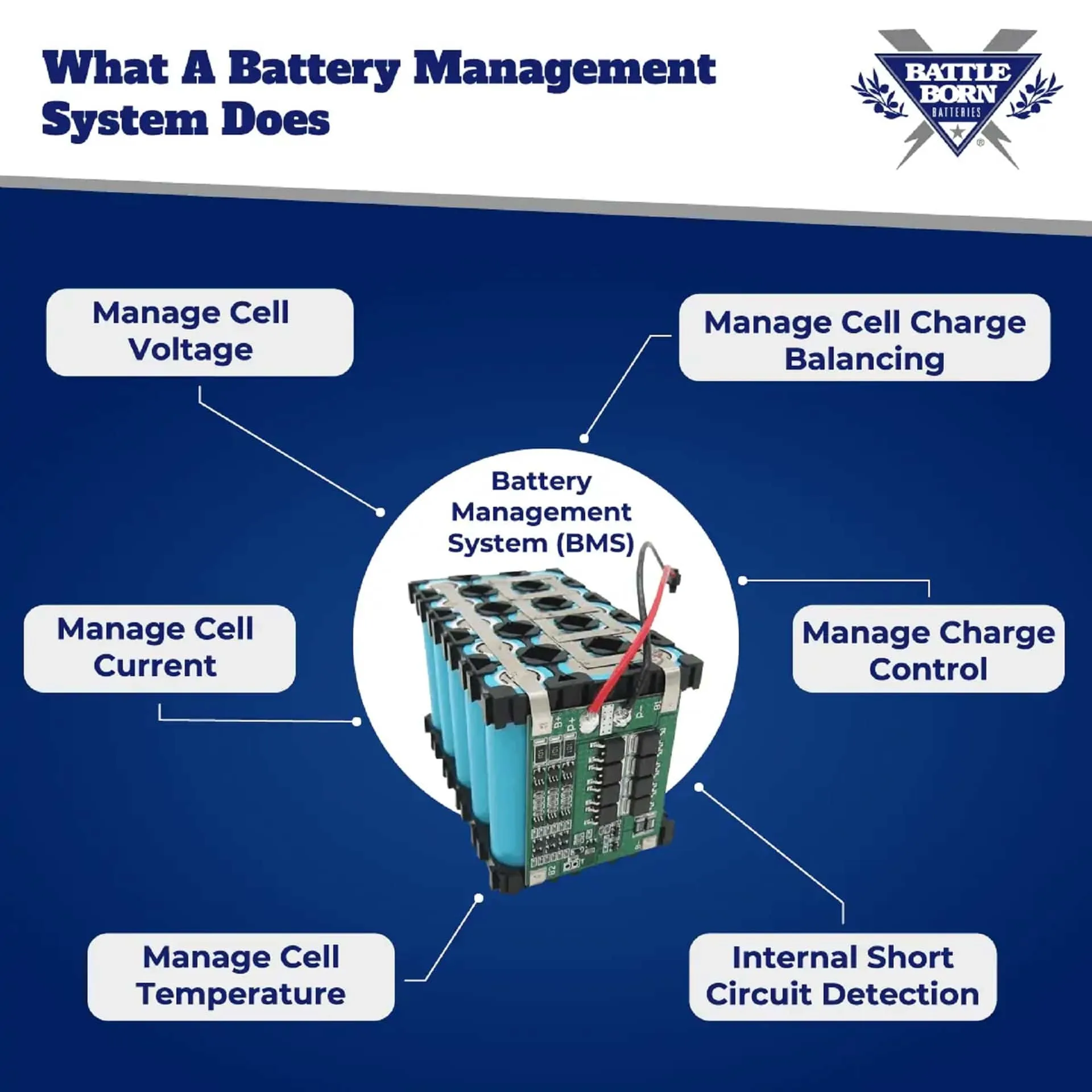
What Is the Function of a Battery Management System? The RVgeeks
What Are The 2 Main Functions Of A Battery This is known as electrochemistry and the system that underpins a battery is. Whether a traditional disposable battery (e.g., aa) or a rechargeable. A battery is a device that stores chemical energy, and converts it to electricity. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). This is known as electrochemistry and the system that underpins a battery is. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. Batteries power our lives by transforming energy from one type to another. When you put those batteries into the flashlight and. To accept and release energy, a battery is coupled to. We need to understand some fundamentals of how electricity works before we can understand the battery. Firstly, electricity is the flow of. Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the.
From www.linkedin.com
BMS in Lithium Batteries What Are Its Main Functions? What Are The 2 Main Functions Of A Battery When you put those batteries into the flashlight and. Two terminals made of different chemicals (typically metals), the anode and the. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. Firstly, electricity is the flow of. Batteries power our lives by transforming energy from one type to another. To accept and release. What Are The 2 Main Functions Of A Battery.
From blog.battery-solution.com
What is liion battery and its component? ⋆ PBS Battery Solution What Are The 2 Main Functions Of A Battery Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. Batteries power our lives by transforming energy from one type to another. Two terminals made of different chemicals (typically metals), the anode and the. We need to understand some fundamentals of how electricity works before we can understand the battery.. What Are The 2 Main Functions Of A Battery.
From www.britannica.com
Battery Composition, Types, & Uses Britannica What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. This is known as electrochemistry and the system that underpins a battery is. Firstly, electricity is the flow of. To accept and release energy, a battery is. What Are The 2 Main Functions Of A Battery.
From theconversation.com
How do lithiumion batteries work? What Are The 2 Main Functions Of A Battery A battery is a device that stores chemical energy, and converts it to electricity. Two terminals made of different chemicals (typically metals), the anode and the. Batteries power our lives by transforming energy from one type to another. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. We need to understand some. What Are The 2 Main Functions Of A Battery.
From schematicfixlankier.z21.web.core.windows.net
Battery In Diagram What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Two terminals made of different chemicals (typically metals), the anode and the. Whether a traditional disposable battery (e.g., aa) or a rechargeable. To accept and release energy, a battery is coupled to. When you put those batteries into the flashlight and. Batteries power our. What Are The 2 Main Functions Of A Battery.
From exowyzmfm.blob.core.windows.net
What Are The Three Main Parts Of A Battery at Connie Welch blog What Are The 2 Main Functions Of A Battery Batteries power our lives by transforming energy from one type to another. There are three main components of a battery: Two terminals made of different chemicals (typically metals), the anode and the. To accept and release energy, a battery is coupled to. When you put those batteries into the flashlight and. We need to understand some fundamentals of how electricity. What Are The 2 Main Functions Of A Battery.
From www.youtube.com
How lithium ion battery works Working principle YouTube What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). There are three main components of a battery: Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a. What Are The 2 Main Functions Of A Battery.
From www.mechanicalbooster.com
How Battery Ignition System Works? Mechanical Booster What Are The 2 Main Functions Of A Battery When you put those batteries into the flashlight and. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). A battery is a device that stores chemical energy, and converts it to electricity. To accept and release energy, a battery is coupled to. There are three main components of a battery: Two terminals made. What Are The 2 Main Functions Of A Battery.
From thepowerfacts.com
What is the Function of Batteries? (Fully Described) The Power Facts What Are The 2 Main Functions Of A Battery This is known as electrochemistry and the system that underpins a battery is. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. Whether a traditional disposable battery (e.g., aa) or a rechargeable. Two terminals made of different chemicals (typically metals), the anode and the. When you put those batteries into the flashlight. What Are The 2 Main Functions Of A Battery.
From www.youtube.com
How A Battery Works YouTube What Are The 2 Main Functions Of A Battery Firstly, electricity is the flow of. We need to understand some fundamentals of how electricity works before we can understand the battery. There are three main components of a battery: This is known as electrochemistry and the system that underpins a battery is. A battery is a device that stores chemical energy, and converts it to electricity. Batteries consist of. What Are The 2 Main Functions Of A Battery.
From www.slideserve.com
PPT AUTOMOTIVE BATTERIES PowerPoint Presentation, free download ID What Are The 2 Main Functions Of A Battery Firstly, electricity is the flow of. We need to understand some fundamentals of how electricity works before we can understand the battery. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. When you put those batteries into the flashlight and. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of. What Are The 2 Main Functions Of A Battery.
From www.thervgeeks.com
What Is the Function of a Battery Management System? The RVgeeks What Are The 2 Main Functions Of A Battery Batteries power our lives by transforming energy from one type to another. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). A battery is a device that stores chemical energy, and converts it to electricity. Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called. What Are The 2 Main Functions Of A Battery.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry What Are The 2 Main Functions Of A Battery This is known as electrochemistry and the system that underpins a battery is. To accept and release energy, a battery is coupled to. We need to understand some fundamentals of how electricity works before we can understand the battery. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. When you put those. What Are The 2 Main Functions Of A Battery.
From maker.pro
Lithiumion Battery Basics Advantages and Applications Maker Pro What Are The 2 Main Functions Of A Battery To accept and release energy, a battery is coupled to. Firstly, electricity is the flow of. A battery is a device that stores chemical energy, and converts it to electricity. We need to understand some fundamentals of how electricity works before we can understand the battery. Batteries consist of two electrical terminals called the cathode and the anode, separated by. What Are The 2 Main Functions Of A Battery.
From www.youtube.com
How does a battery works YouTube What Are The 2 Main Functions Of A Battery To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. A battery is a device that stores chemical energy, and converts it to electricity. Firstly, electricity is the flow of. To accept and release energy, a battery is coupled to. Batteries consist of two electrical terminals called the cathode and the anode, separated. What Are The 2 Main Functions Of A Battery.
From wiremanualkerstin99.z19.web.core.windows.net
Car Battery Cell Diagram What Are The 2 Main Functions Of A Battery Batteries power our lives by transforming energy from one type to another. Whether a traditional disposable battery (e.g., aa) or a rechargeable. We need to understand some fundamentals of how electricity works before we can understand the battery. There are three main components of a battery: Batteries consist of two electrical terminals called the cathode and the anode, separated by. What Are The 2 Main Functions Of A Battery.
From thepowerfacts.com
What are the Functions of a Battery? (Answered) The Power Facts What Are The 2 Main Functions Of A Battery Two terminals made of different chemicals (typically metals), the anode and the. This is known as electrochemistry and the system that underpins a battery is. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Batteries power our lives by transforming energy from one type to another. There are three main components of a. What Are The 2 Main Functions Of A Battery.
From www.takomabattery.com
Main function of battery management system The Best lithium ion battery What Are The 2 Main Functions Of A Battery Two terminals made of different chemicals (typically metals), the anode and the. A battery is a device that stores chemical energy, and converts it to electricity. Firstly, electricity is the flow of. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Whether a traditional disposable battery (e.g., aa) or a rechargeable. This is. What Are The 2 Main Functions Of A Battery.
From letstalkscience.ca
How does a lithiumIon battery work? Let's Talk Science What Are The 2 Main Functions Of A Battery Two terminals made of different chemicals (typically metals), the anode and the. We need to understand some fundamentals of how electricity works before we can understand the battery. To accept and release energy, a battery is coupled to. Firstly, electricity is the flow of. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy).. What Are The 2 Main Functions Of A Battery.
From wiringfixhovers.z13.web.core.windows.net
Simple Diagram Of A Battery What Are The 2 Main Functions Of A Battery We need to understand some fundamentals of how electricity works before we can understand the battery. To accept and release energy, a battery is coupled to. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Whether a traditional disposable battery (e.g., aa) or a rechargeable. There are three main components of a battery:. What Are The 2 Main Functions Of A Battery.
From www.thetechnologynet.com
Secondary Battery Definition, uses, and Types Of And More What Are The 2 Main Functions Of A Battery Batteries power our lives by transforming energy from one type to another. There are three main components of a battery: A battery is a device that stores chemical energy, and converts it to electricity. When you put those batteries into the flashlight and. To accept and release energy, a battery is coupled to. To envision how a battery works, picture. What Are The 2 Main Functions Of A Battery.
From www.slideserve.com
PPT Battery Basics PowerPoint Presentation, free download ID2339466 What Are The 2 Main Functions Of A Battery Firstly, electricity is the flow of. To accept and release energy, a battery is coupled to. Two terminals made of different chemicals (typically metals), the anode and the. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. A battery is a device that stores chemical energy, and converts it to electricity. We. What Are The 2 Main Functions Of A Battery.
From www.youtube.com
How a lead acid battery works YouTube What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. There are three main components of a battery: This is known as electrochemistry and the system that underpins a battery is. Two terminals made. What Are The 2 Main Functions Of A Battery.
From guidepartgail.z21.web.core.windows.net
Electric Diagram Of A Battery What Are The 2 Main Functions Of A Battery Firstly, electricity is the flow of. There are three main components of a battery: A battery is a device that stores chemical energy, and converts it to electricity. Whether a traditional disposable battery (e.g., aa) or a rechargeable. To accept and release energy, a battery is coupled to. Batteries power our lives by transforming energy from one type to another.. What Are The 2 Main Functions Of A Battery.
From stock.adobe.com
A schematic showing the difference between series and parallel battery What Are The 2 Main Functions Of A Battery This is known as electrochemistry and the system that underpins a battery is. To accept and release energy, a battery is coupled to. A battery is a device that stores chemical energy, and converts it to electricity. Whether a traditional disposable battery (e.g., aa) or a rechargeable. Two terminals made of different chemicals (typically metals), the anode and the. Batteries. What Are The 2 Main Functions Of A Battery.
From www.wiringdraw.com
What Is The Function Of A Battery In Simple Circuit Wiring Draw And What Are The 2 Main Functions Of A Battery Batteries power our lives by transforming energy from one type to another. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Firstly, electricity is the flow of. This is known as electrochemistry and the system that. What Are The 2 Main Functions Of A Battery.
From www.changfi.com
วิธีการซ่อมแบตเตอรี่ตะกั่วกรด ช่างไฟดอทคอม What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. We need to understand some fundamentals of how electricity works before we can understand the battery. To envision how a battery works, picture yourself. What Are The 2 Main Functions Of A Battery.
From www.takomabattery.com
What is an electrolyte a component of battery The Best lithium ion What Are The 2 Main Functions Of A Battery There are three main components of a battery: Firstly, electricity is the flow of. Two terminals made of different chemicals (typically metals), the anode and the. Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. Batteries power our lives by transforming energy from one type to another. To accept. What Are The 2 Main Functions Of A Battery.
From batteryguy.com
What are Alkaline Batteries? Knowledge Base What Are The 2 Main Functions Of A Battery To accept and release energy, a battery is coupled to. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). When you put those batteries into the flashlight and. There are three main components of a battery: Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material. What Are The 2 Main Functions Of A Battery.
From www.researchgate.net
The principle of the lithiumion battery (LiB) showing the What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). Whether a traditional disposable battery (e.g., aa) or a rechargeable. To accept and release energy, a battery is coupled to. Two terminals made of different chemicals (typically metals), the anode and the. Batteries power our lives by transforming energy from one type to another.. What Are The 2 Main Functions Of A Battery.
From stock.adobe.com
Vecteur Stock Dry cell battery infographic diagram parts structure What Are The 2 Main Functions Of A Battery To accept and release energy, a battery is coupled to. Firstly, electricity is the flow of. Batteries power our lives by transforming energy from one type to another. A battery is a device that stores chemical energy, and converts it to electricity. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). To envision. What Are The 2 Main Functions Of A Battery.
From www.alamy.com
Simple battery scheme. The illustration shows the main elements of What Are The 2 Main Functions Of A Battery We need to understand some fundamentals of how electricity works before we can understand the battery. Batteries power our lives by transforming energy from one type to another. To accept and release energy, a battery is coupled to. To envision how a battery works, picture yourself putting alkaline batteries, like double aas, into a flashlight. This is known as electrochemistry. What Are The 2 Main Functions Of A Battery.
From www.livescience.com
How Do Batteries Work? Live Science What Are The 2 Main Functions Of A Battery Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). There are three main components of a battery: Batteries consist of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. This is known as electrochemistry and the system that underpins a battery is. To accept and. What Are The 2 Main Functions Of A Battery.
From cosmosmagazine.com
How do batteries work? What Are The 2 Main Functions Of A Battery Two terminals made of different chemicals (typically metals), the anode and the. We need to understand some fundamentals of how electricity works before we can understand the battery. Firstly, electricity is the flow of. Batteries come in all different shapes, sizes, voltages, and capacities (amounts of stored charge or energy). There are three main components of a battery: Whether a. What Are The 2 Main Functions Of A Battery.
From www.science-sparks.com
How do batteries work? What Are The 2 Main Functions Of A Battery We need to understand some fundamentals of how electricity works before we can understand the battery. This is known as electrochemistry and the system that underpins a battery is. When you put those batteries into the flashlight and. Whether a traditional disposable battery (e.g., aa) or a rechargeable. To envision how a battery works, picture yourself putting alkaline batteries, like. What Are The 2 Main Functions Of A Battery.